2. 遵义医科大学基础药理教育部重点实验室暨特色民族药教育部国际合作联合实验室,遵义 563000;
3. 遵义医科大学药学院,遵义 563000;
4. 遵义医科大学附属医院早期临床研究病房,遵义 563000
2. Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi 563000, China;
3. School of Pharmacy, Zunyi Medical University, Zunyi 563000, China;
4. Early Clinical Research Ward, Affiliated Hospital of Zunyi Medical University, Zunyi 563000, China
辐射治疗在多种胸部恶性肿瘤的局部治疗中扮演着重要角色,然而,在放疗过程中,心脏不可避免受到射线照射,严重者导致心脏损伤(RICD)。RICD的发生机制可能与磷脂酰肌醇3激酶/蛋白激酶B(PI3K/AKT)信号通路的调控有关,该通路在增加心肌细胞凋亡中起作用。RICD影响患者预后,目前尚缺乏针对RICD有效的防治药物[1]。淫羊藿苷(ICA)是民族中草药淫羊藿的主要活性成分,能够减轻心肌细胞凋亡,在多种心血管疾病中有明显的保护作用,并具有不良反应轻的特点[2],但其是否有预防RICD的作用尚不清楚。本研究采用C57BL/6J小鼠,单次30 Gy的心脏照射构建RICD模型,评估淫羊藿苷对RICD的防治效果,并探索其潜在机制。
材料与方法1. 主要试剂与仪器:淫羊藿苷(南通飞宇生物科技有限公司);小鼠心肌肌钙蛋白、肌酸激酶同工酶MB酶联免疫吸附测定试剂盒(武汉伊莱瑞特生物科技股份有限公司);TRIzol、PrimeScript RT试剂盒(TaKaRa-Bio公司,日本);实时荧光定量PCR引物合成于公司上海生物工程有限公司;RIPA蛋白裂解液(Abcam公司,英国);Caspase-3抗体购于美国Santa Cruz公司;Bcl-2抗体、Bax抗体购于杭州华安生物技术有限公司;Cleaved Caspase-3抗体、P-PI3K抗体、PI3K抗体、P-Akt抗体、Akt抗体、β-肌动蛋白抗体购自美国CST公司;直线加速器(Elekta公司,瑞典);Vevo2100超声成像系统(VisualSonics公司,加拿大);酶标仪(Thermo Fisher公司,美国);聚合酶链式反应PCR仪T100 Thermal cycle、CFX Connet型荧光定量PCR仪、化学发光成像系统、电泳仪(Bio-rad公司,美国)。
2. 实验动物及照射条件:6~8周龄雌性C57BL/6J小鼠48只,购自遵义医科大学动物中心[合格证号SCXK-(黔)2021-00002]。于标准条件(温度18~22℃,湿度40%~60%,12 h昼/12 h夜周期)下饲养,已获遵义医科大学实验动物福利伦理委员会批准(伦理审批号:ZMU21-2306-062)。适应性饲养1周后,按随机数表法将小鼠分为对照(CON)组、照射(IR)组和照射联合淫羊藿苷(IR+ICA)组,每组16只。用0.5%羧甲基纤维素钠溶液配制成7 mg/ml的淫羊藿苷混悬液,每日灌胃IR+ICA组小鼠(70 mg·kg-1·d-1),对照组和IR组灌胃等体积的0.5%羧甲基纤维素钠溶液,每日记录小鼠体重、精神、进食及活动状况。灌胃2周后,用1%戊巴比妥钠(75 mg/kg)腹腔注射麻醉小鼠,固定于特制模具上,行30 Gy心脏调强辐射处理(6 MV X射线,剂量率600 cGy/min,单次照射源皮距97.54 cm)。若体重下降超过20%或出现意识障碍,麻醉处死并取材。
3. 血清心肌标志物检测:照后2周,采集每只小鼠颌下静脉血约100 μl,室温放置1 h后1 000 g离心20 min取上清。按小鼠肌酸激酶同工酶(CK-MB)酶联免疫吸附测定试剂盒、肌钙蛋白(cTnT/TNNT2)酶联免疫吸附测定试剂盒使用说明书进行。
4. 小鼠心脏超声检查:照后12周,使用Vevo2100超声成像系统进行心脏超声检查,用1%戊巴比妥钠(75 mg/kg)腹腔注射麻醉小鼠,脱毛处理小鼠的胸腹部,并固定在检查托架上,在心前区涂擦耦合剂后进行检查,测量左室射血分数(EF)、心室短轴缩短率(FS)及左心室舒张期末内径(LVDD)。
5. 组织病理学检查:照后12周,麻醉处死全部小鼠,取心肌组织,4%甲醛固定,石蜡包埋,切取4 μm厚切片,苏木精-伊红(HE)染色评估心肌细胞的结构完整性、细胞核形态及炎症细胞的浸润情况,Masson染色评估心肌纤维化的程度和分布,在光镜下观察,用Image J进行心肌胶原面积半定量分析,心肌胶原容积分数(CVF)=视野中胶原面积/视野总面积。
6. 实时定量反转录聚合酶链反应(real-time RT-PCR)检测脑钠肽(BNP)、转化生长因子β(TGF-β)及白细胞介素6(IL-6)相对基因表达量:从小鼠心肌组织中提取总RNA,使用PrimeScript RT试剂盒反转录为cDNA,设计合成针对BNP、TGF-β及IL-6的real-time RT-PCR引物,引物序列见表 1。按说明书配制反应体系并在real\|time PCR仪上进行扩增。ΔCt =目的基因Ct值-内参基因(GAPDH)Ct值,ΔΔCt = ΔCt实验组-ΔCt对照组,目的基因相对表达量(RQ)=2-ΔΔCt。
|
|
表 1 实时定量PCR引物序列 Table 1 Primer sequences of quantitative real-time PCR (qRT-PCR) |
7. 蛋白质印迹法(western blot)检测凋亡、PI3K/Akt通路相关蛋白:称取新鲜小鼠心肌组织20 mg,加入300 μl蛋白裂解液,于冰上进行超声破碎,Bradford法测定蛋白质浓度,取30 μg蛋白质与5 × loading buffer 4∶1混合加热变性备用,配制12.5%十二烷基磺酸钠-聚丙烯酰氨凝胶(SDS-PAGE)分离胶和5% SDS-PAGE积聚胶,恒压电泳结束后,将蛋白在低温、恒流下湿转至聚偏二氟乙烯(PVDF)膜上,5% BSA室温封闭2 h,一抗4℃孵育过夜,辣根过氧化标记的二抗室温孵育1 h,电化学发光检测,ChemiDocTM Touch成像系统显影,检测caspase-3、cleaved caspase-3、Bcl-2、Bax、P-PI3K、PI3K、P-Akt、Akt表达,β-肌动蛋白作为内参,Image J进行半定量分析。
8. 统计学处理:使用GraphPad Prism 8.0进行作图和统计分析。采用Kaplan-Meier方法绘制生存曲线,Log-rank检验比较组间生存差异。对于符合正态分布且方差齐的数据,以x±s表示。采用独立样本t检验进行组间比较。P < 0.05为差异有统计学意义。
结果1. 淫羊藿苷改善RICD小鼠体重下降及生存率:CON组小鼠精神和活动正常,体重随着周龄增长逐渐上升。IR组小鼠在受照射后出现精神萎靡,进食及活动量下降,体重减轻,且在照射后1周时达到最低点,明显低于CON组(t = 2.46,P < 0.05)。IR+ICA组小鼠在照射后精神状态尚可,活动、进食量及体重下降程度较IR组轻微。此后IR组和IR+ICA组小鼠的精神状态、进食、活动量恢复,体重逐渐增加。但照后12周,IR组小鼠平均体重低于CON组(t = 2.47,P < 0.05),而IR+ICA组小鼠平均体重高于IR组(t = 5.13,P < 0.001)。CON组小鼠全部存活,而IR组在放疗后2周内有部分小鼠死亡,生存率降低(HR = 8.25, 95%CI:1.157~58.770, P < 0.05)。IR+ICA组则未出现死亡小鼠,较IR组提高(HR = 0.12, 95%CI:0.017~0.864, P < 0.05)(图 1)。
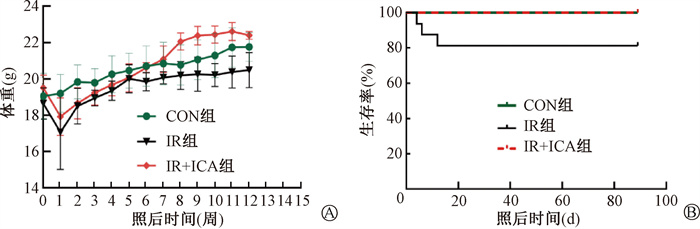
|
图 1 淫羊藿苷对辐射诱导心脏损伤小鼠体重(A)、生存情况(B)的影响 Figure 1 Effects of icariin on body weight(A) and survival(B) in mice with RICD |
2. 淫羊藿苷降低RICD小鼠血清心肌标志物水平:小鼠受照2周,IR组小鼠血清的CK-MB和cTnT水平较对照组均升高(t = 5.28、8.89,P < 0.05);经淫羊藿苷处理组,上述指标均较IR组降低(t = 3.24、4.81,P < 0.05),提示淫羊藿苷可在一定程度上减轻由辐射引起的小鼠心肌标志物异常升高,具有潜在的保护作用(表 2)。
|
|
表 2 淫羊藿苷对辐射诱导心脏损伤小鼠心肌标志物的影响(x±s) Table 2 Effects of icariin on cardiac biomarkers in mice with radiation-induced cardiac disease(x±s) |
3. 淫羊藿苷改善RICD小鼠心功能:照射后第12周,IR组小鼠的左室壁运动欠协调,而IR+ICA组的左室壁运动协调性较IR组有所改善。与CON组相比,IR组小鼠的EF和FS显著下降(t = 7.02、6.51,P < 0.01),LVDD增加(t = 4.45,P < 0.05)。IR+ICA组小鼠的EF和FS值较IR组升高(t = 3.23、3.05,P < 0.05),LVDD值则降低(t = 3.02,P < 0.05)。综上所述,30 Gy的心脏照射可显著导致小鼠心功能下降,符合辐射性心脏损伤的表现,而经淫羊藿苷处理后,心功能下降得到部分改善(表 3)。
|
|
表 3 淫羊藿苷对辐射诱导心脏损伤小鼠心功能的影响(x±s) Table 3 Effects of icariin on cardiac ultrasound parameters and myocardial collagen volume fraction in mice with radiation-induced cardiac disease(x±s) |
4. 淫羊藿苷对RICD小鼠心肌病理学的影响:HE染色提示IR组HE染色部分心肌细胞出现水肿和排列紊乱,肌纤维部分断裂。Masson染色结果显示IR组心肌间质中有胶原纤维沉积,较CON组明显增加,表明辐射性心脏损伤模型成功建立。而IR+ICA组心肌细胞轻度肿胀,排列较为整齐。观察到仅有少量胶原纤维散在分布于心肌细胞间质中,相较于IR组明显减少(图 2)。经分析各组小鼠的CVF,IR组明显高于CON组(t = 8.70,P < 0.001),而灌胃ICA后CVF较IR组显著降低(t = 4.77,P < 0.01),见表 3。表明淫羊藿苷改善了由辐射引起的心脏病理损伤,减轻了其纤维化程度。
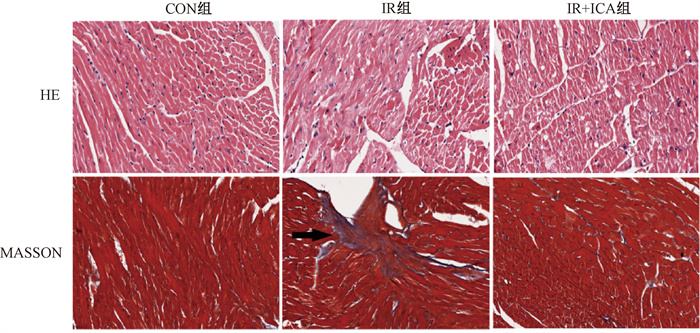
|
注:箭头指示为胶原沉积最明显的区域 图 2 淫羊藿苷对辐射诱导心脏损伤小鼠心肌病理学的影响 ×640 Figure 2 Effects of icariin on myocardial pathology of mice with RICD ×640 |
5. 淫羊藿苷降低RICD小鼠BNP、TGF-β、IL-6基因相对表达:与CON组相比,IR组小鼠心脏BNP、TGF-β和IL-6的相对表达水平增加,差异有统计学意义(t = 4.23、6.39、4.61,P < 0.05)。淫羊藿苷处理可改善上述情况,IR+ICA组中BNP、TGF-β和IL-6的相对表达量较IR组降低(t =2.83、4.15、2.96,P < 0.05)。这表明淫羊藿苷有效降低了辐射引起的心衰标志物、促纤维化细胞因子、炎症标志物水平(表 4)。
|
|
表 4 淫羊藿苷对辐射诱导心脏损伤小鼠心肌BNP、TGF-β和IL-6基因表达的影响(x±s) Table 4 Effects of icariin on the gene expression of BNP, TGF-β, and IL-6 in cardiomyocytes for mice with RICD (x±s) |
6. 淫羊藿苷可减轻心肌细胞凋亡和激活PI3K/Akt信号通路:照射后小鼠心肌细胞中促进凋亡的Cleaved Caspase-3、Bax蛋白明显增加(t = 6.29、9.54,P < 0.05),抑制凋亡的Bcl-2蛋白表达降低(t = 8.20,P < 0.001)。上述蛋白在灌胃ICA后出现逆转,Cleaved Caspase-3、Bax相对IR组明显降低(t = 3.23、3.24,P < 0.05),Bcl-2蛋白较IR组表达水平增加(t = 2.92,P < 0.05)。进一步检测各组心肌PI3K/Akt的磷酸化水平,发现IR抑制了心肌PI3K和Akt磷酸化(t = 6.47、3.42,P < 0.001),而灌胃ICA部分恢复了IR处理后小鼠PI3K和Akt的磷酸化水平(t = 2.89、8.35,P < 0.001)。因此,推测ICA可能通过上调PI3K和Akt的磷酸化水平来缓解IR诱导的小鼠心肌细胞凋亡(图 3、表 5)

|
图 3 淫羊藿苷对辐射诱导心脏损伤小鼠心肌凋亡、PI3K/Akt通路蛋白的影响 A.心肌凋亡相关蛋白表达;B.PI3K/Akt通路相关蛋白表达 Figure 3 Effects of icariin on the expression of cardiomyocyte apoptosis-related proteins and proteins associated with PI3K/Akt pathway in mice with RICD A. Cardiomyocyte apoptosis-related proteins; B. Expression of proteins associated with PI3K/Akt pathway |
|
|
表 5 淫羊藿苷对辐射诱导心脏损伤小鼠心肌凋亡、PI3K/Akt通路蛋白表达灰度分析的影响(x±s) Table 5 Effect of icariin on grayscale analysis of myocardial apoptosis and PI3K/Akt pathway protein expression in mice with radiation-induced cardiac injury(x±s) |
讨论
辐射治疗是肺癌、乳腺癌、食管癌及淋巴瘤等多种胸部恶性肿瘤的主要局部治疗手段。然而,在胸部辐射治疗过程中,心脏将不可避免地受到辐射线的影响,并可能诱导RICD的发生。由于恶性肿瘤患者生存期短,患者往往在RICD的潜伏期内便因肿瘤本身而死亡,导致RICD一直未引起足够的重视[3-4]。近年来,随着新兴治疗技术及药物的不断涌现,恶性肿瘤患者生存期不断延长,与辐射治疗相关的心脏损伤逐渐显现,迫使人们不得不重新审视RICD。一项乳腺癌相关研究表明,RICD与心脏所接受的平均剂量(Dmean)呈线性相关,Dmean每提高1 Gy,患者心脏相关累积死亡风险增加3%[5]。美国肿瘤放射治疗协作组(Radiation Therapy Oncology Group, RTOG)0617研究则表明,与60 Gy相比,接受高剂量74 Gy辐射治疗并不能提高局部晚期患者的长期生存[6]。进一步分析表明,对Ⅲ期非小细胞肺癌(NSCLC)患者而言,较高的心脏剂量与较短的总生存期相关,也是限制局部晚期NSCLC放疗剂量提升的主要障碍之一[7]。上述结果均提示,RICD可能在患者生存和预后中扮演着重要的角色,因此,临床迫切需要寻找防治RICD的可行方案。
当前,恶性肿瘤常规的辐射治疗方式为1.8~2.2 Gy/次的累积照射,在既往的探索中,有研究者用这种更具临床相关性的分割方案对小鼠进行左心照射,但模型未能建立成功[8]。Schlaak等[9]和Ghita-Pettigrew等[10]的研究发现,单次高剂量照射方案可以在小鼠中诱导更严重的RICD。而临床研究也显示,当患者心包受照剂量>26.1 Gy时,发生心包积液的风险明显增加[11]。暴露于30 Gy及以上剂量的患者,发生心肌变性和纤维化的风险显著增加[12-13]。为提高建模成功率、控制建模成本,单次高剂量照射是构建RICD动物模型的常用方法,剂量高者可予30 Gy及以上[14-15]。据此,为更好地评估淫羊藿苷对RICD的保护作用,本研究采用单次30 Gy对C57BL/6J雌性小鼠进行全心照射以构建RICD模型。结果发现,与CON组相比,IR组小鼠体重下降,部分小鼠死亡。照后2周,IR组血清CK-MB、cTnT水平较CON组明显升高。照后12周,心脏彩超提示IR组小鼠心功能不全,心肌病理检查表明IR组小鼠部分心肌细胞水肿、排列紊乱,心肌纤维沉积。心肌心衰标志物BNP、促纤维化细胞因子TGF-β、促炎细胞因子IL-6基因的表达也上调。上述结果表明,小鼠30 Gy单次全心照射诱导RICD模型建立成功。
民族中草药淫羊藿主要产于贵州、甘肃等省,淫羊藿苷作为其主要活性成分之一,在心血管保护、免疫调节等领域具有广泛的药理活性[2, 16]。已有研究表明,淫羊藿苷及其代谢产物通过激活PI3K/Akt等信号通路减轻心肌细胞凋亡,预防高心病、缓解充血性心衰[17-18]。此外,淫羊藿苷还可以抑制内质网氧化应激减轻内皮祖细胞自噬,减轻血管内皮功能障碍[19-20],激活NO/cGMP信号通路可抑制血管细胞增殖,改善血管重塑[21]。还可通过上调sirtuin 6酶活性和抑制-κB通路以干预心脏炎症[22]。在临床中,淫羊藿苷用于动脉粥样硬化、心力衰竭[2]等疾病,且具有抗肿瘤效应,目前主要应用于乳腺癌、肝癌的治疗[23-24]。因此,淫羊藿苷可能同时给胸部肿瘤患者带来多途径的获益,具有广泛的应用前景。有研究指出,RICD的发生可能与PI3K/Akt信号通路的抑制、心肌细胞凋亡增加有关[25-26]。正常状态下,激活的PI3K/Akt信号通路可以催化PIP2转化为PIP3,激活Akt,通过磷酸化mTOR等一系列下游靶蛋白,从而促进细胞存活并抑制细胞凋亡。PI3K/Akt信号通路还参与调控细胞代谢、血管稳态、血栓形成、心肌纤维化等过程,其失调在多种心脏疾病的发病机制中被证明起到了关键作用[27]。本研究系统评估了淫羊藿苷对RICD的保护作用,揭示其能够降低RICD小鼠血清心肌标志物水平,改善其心功能,降低心肌纤维化程度,减轻了照射导致的心脏病理损害。淫羊藿苷作为一种具有多种治疗效应的中草药成分,具有广泛的临床应用前景。此外,本研究探讨了淫羊藿苷可能通过激活PI3K/Akt信号通路抑制心肌细胞凋亡的分子机制,为RICD的防治提供了新的思路。
综上所述,本研究证实了单次30 Gy剂量的心脏照射可成功构建小鼠RICD模型,而淫羊藿苷对于RICD小鼠模型具有保护作用,具备一定的临床探索价值,值得深入研究。
利益冲突 无
作者贡献声明 尹凤敏负责设计研究方案,研究实施及论文撰写;蒲超元、苏紫璇、吴梦嘉、罗欣怡负责动物研究实施;冉涛、张雷、刘其林、陈言负责数据分析、论文整理;龚其海负责部分实验设计;胡威负责提出研究思路、设计研究方案、技术指导及论文修改
| [1] |
Jin JY, Hu C, Xiao Y, et al. Higher radiation dose to the immune cells correlates with worse tumor control and overall survival in patients with stage Ⅲ NSCLC: A secondary analysis of RTOG0617[J]. Cancers (Basel), 2021, 13(24): 6193. DOI:10.3390/cancers13246193 |
| [2] |
Zeng Y, Xiong Y, Yang T, et al. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms[J]. Biomed Pharmacother, 2022, 147: 112642. DOI:10.1016/j.biopha.2022.112642 |
| [3] |
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer[J]. N Engl J Med, 2013, 368(11): 987-998. DOI:10.1056/NEJMoa1209825 |
| [4] |
王军, 武亚晶. 放射性心脏损伤研究进展[J]. 中华放射肿瘤学杂志, 2019, 28(10): 721-727. Wang J, Wu YJ. Research progress on radiation-induced heart damage—basic mechanism[J]. Chin J Radiat Oncol, 2019, 28(10): 721-727. DOI:10.3760/cma.j.issn.1004-4221.2019.10.001 |
| [5] |
Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects[J]. Int J Radiat Oncol Biol Phys, 2010, 76(3): 656-665. DOI:10.1016/j.ijrobp.2009.09.064 |
| [6] |
Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage ⅢA or ⅢB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study[J]. Lancet Oncol, 2015, 16(2): 187-199. DOI:10.1016/S1470-2045(14)71207-0 |
| [7] |
Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage Ⅲ non-small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy[J]. J Clin Oncol, 2017, 35(13): 1387-1394. DOI:10.1200/JCO.2016.70.0229 |
| [8] |
Lee CL, Lee JW, Daniel AR, et al. Characterization of cardiovascular injury in mice following partial-heart irradiation with clinically relevant dose and fractionation[J]. Radiother Oncol, 2021, 157: 155-162. DOI:10.1016/j.radonc.2021.01.023 |
| [9] |
Schlaak RA, Frei A, Fish BL, et al. Acquired immunity is not essential for radiation-induced heart dysfunction but exerts a complex impact on injury[J]. Cancers (Basel), 2020, 12(4): 983. DOI:10.3390/cancers12040983 |
| [10] |
Ghita-Pettigrew M, Edgar KS, Kuburas R, et al. Dose-dependent changes in cardiac function, strain and remodelling in a preclinical model of heart base irradiation[J]. Radiother Oncol, 2024, 193: 110113. DOI:10.1016/j.radonc.2024.110113 |
| [11] |
Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy[J]. Int J Radiat Oncol Biol Phys, 2008, 70(3): 707-714. DOI:10.1016/j.ijrobp.2007.10.056 |
| [12] |
Brosius FC 3rd, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3500 rads to the heart[J]. Am J Med, 1981, 70(3): 519-530. DOI:10.1016/0002-9343(81)90574-x |
| [13] |
Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases[J]. Hum Pathol, 1996, 27(8): 766-773. DOI:10.1016/s0046-8177(96)90447-5 |
| [14] |
Walls GM, O'Kane R, Ghita M, et al. Murine models of radiation cardiotoxicity: A systematic review and recommendations for future studies[J]. Radiother Oncol, 2022, 173: 19-31. DOI:10.1016/j.radonc.2022.04.030 |
| [15] |
李鹏, 陈凡, 孙祥益, 等. 放射性心脏损伤动物模型相关实验研究进展[J]. 中国辐射卫生, 2020, 29(1): 93-97. Li P, Chen F, Sun XY, et al. Progress in experimental studies on animal models of radiation-induced heart injury[J]. Chin J Radiol Health, 2020, 29(1): 93-97. DOI:10.13491/j.issn.1004-714X.2020.01.022 |
| [16] |
Wu B, Feng JY, Yu LM, et al. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage[J]. Br J Pharmacol, 2018, 175(21): 4137-4153. DOI:10.1111/bph.14457 |
| [17] |
Song YH, Cai H, Gu N, et al. Icariin attenuates cardiac remodelling through down-regulating myocardial apoptosis and matrix metalloproteinase activity in rats with congestive heart failure[J]. J Pharm Pharmacol, 2011, 63(4): 541-549. DOI:10.1111/j.2042-7158.2010.01241.x |
| [18] |
Wu Y, Yue Y, Fu S, et al. Icariside Ⅱ prevents hypertensive heart disease by alleviating endoplasmic reticulum stress via the PERK/ATF-4/CHOP signalling pathway in spontaneously hypertensive rats[J]. J Pharm Pharmacol, 2019, 71(3): 400-407. DOI:10.1111/jphp.13041 |
| [19] |
Tang Y, Jacobi A, Vater C, et al. Icariin promotes angiogenic differentiation and prevents oxidative stress-induced autophagy in endothelial progenitor cells[J]. Stem Cells, 2015, 33(6): 1863-1877. DOI:10.1002/stem.2005 |
| [20] |
Wang FY, Jia J, Song HH, et al. Icariin protects vascular endothelial cells from oxidative stress through inhibiting endoplasmic reticulum stress[J]. J Integr Med, 2019, 17(3): 205-212. DOI:10.1016/j.joim.2019.01.011 |
| [21] |
Li LS, Luo YM, Liu J, et al. Icariin inhibits pulmonary hypertension induced by monocrotaline through enhancement of NO/cGMP signaling pathway in rats[J]. Evid Based Complement Alternat Med, 2016, 2016: 7915415. DOI:10.1155/2016/7915415 |
| [22] |
Sharma S, Khan V, Dhyani N, et al. Icariin attenuates isoproterenol-induced cardiac toxicity in Wistar rats via modulating cGMP level and -κB signaling cascade[J]. Hum Exp Toxicol, 2020, 39(2): 117-126. DOI:10.1177/0960327119890826 |
| [23] |
Yu Z, Guo J, Hu M, et al. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma[J]. ACS Nano, 2020, 14(4): 4816-4828. DOI:10.1021/acsnano.0c00708 |
| [24] |
Zhao M, Xu P, Shi W, et al. Icariin exerts anti-tumor activity by inducing autophagy via AMPK/mTOR/ULK1 pathway in triple-negative breast cancer[J]. Cancer Cell Int, 2024, 24(1): 74. DOI:10.1186/s12935-024-03266-9 |
| [25] |
Azimzadeh O, Sievert W, Sarioglu H, et al. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction[J]. J Proteome Res, 2015, 14(2): 1203-1219. DOI:10.1021/pr501141b |
| [26] |
Ren C, Wang L, Li X, et al. Elucidating the mechanism of action of radix angelica sinensis (Oliv.) diels and radix astragalus mongholicus bunge ultrafiltration extract on radiation-induced myocardial fibrosis based on network pharmacology and experimental research[J]. Eur J Pharm Sci, 2024, 199: 106794. DOI:10.1016/j.ejps.2024.106794 |
| [27] |
Ghafouri-Fard S, Khanbabapour Sasi A, Hussen BM, et al. Interplay between PI3K/AKT pathway and heart disorders[J]. Mol Biol Rep, 2022, 49(10): 9767-9781. DOI:10.1007/s11033-022-07468-0 |
 2025, Vol. 45
2025, Vol. 45


