血管内皮细胞是位于血液与血管壁之间的单层扁平或多角形细胞,对电离辐射(ionizing radiation,IR)敏感[1]。无论是局部照射还是全身照射,放疗引起的各种正常器官或组织的损伤,都与血管内皮损伤相关。辐射事故回顾病例发现血管内皮细胞损伤与主要器官早期和晚期放射病变密切相关[2]。近年研究发现电离辐射可诱导血管内皮细胞衰老[3]。本文对血管内皮细胞损伤机制、电离辐射诱导细胞早衰以及衰老相关分泌表型(senescence-associated secretory phenotype, SASP)相关早衰在电离辐射诱导血管内皮细胞损伤中的作用等方面进行简要综述,为预防电离辐射诱导血管内皮细胞损伤提供理论支持。
一、辐射诱导血管内皮细胞损伤特点血管内皮细胞对电离辐射相对敏感,电离辐射会导致内皮细胞损伤的发生[4],损伤程度取决于其受照剂量和时间。在0.5~10 Gy剂量下X射线主要导致细胞衰老,而细胞凋亡水平有限[5]。Yentrapalli等[6-7]研究发现,不同低剂量率的γ射线可引起HUVECs细胞发生早衰,并发现激活Akt/PI3K/mTOR、p53/p21等早衰相关信号通路。
辐射诱导的内皮损伤呈双相模式,可在电离辐射暴露后早期及晚期出现。急性期损伤发生在辐射后数小时至数周内,其特征为内皮肿胀,血管通透性改变,淋巴细胞黏附以及浸润和凋亡。辐射后数周至数月会出现晚期血管效应,如毛细血管塌陷、基底膜增厚、瘢痕形成和纤维化[4, 8]。此外,一项广岛和长崎暴露后队列研究表明,低剂量辐射暴露会使中风和心肌梗塞等心血管疾病(cardiovascular diseases,CVD)的发病风险增加[9]。因此,揭示电离辐射引起内皮细胞损伤的机制具有理论和实际意义。
电离辐射引起内皮细胞损伤的机制主要涉及氧化应激和炎症反应等。氧化应激(oxidative stress,OS)是指机体活性氧(reactive oxygen species,ROS)产生过多或机体抗氧化能力降低、氧化系统和抗氧化系统平衡紊乱的过程,可导致潜在性损伤。氧化应激通过氧自由基过氧化反应诱导血管内皮细胞损伤。自由基过氧化反应可导致细胞膜脂质过氧化,细胞转运功能及酶功能障碍,如半胱天冬酶-3(caspase-3)活性变化,诱导内皮细胞死亡,刺激血管内皮细胞合成血小板活化因子,使血小板和中性粒细胞聚集,促进炎症反应[10]。一定剂量的电离辐射可通过氧化应激产生ROS,影响内皮细胞功能。ROS通过激活内皮NO合酶(endothelial nitric oxide synthase,eNOS)直接引起内皮细胞损伤。ROS增加也可激活核转录因子κB(nuclear factor kappa-B,NF-κB)、丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)等信号通路,从而引发内皮细胞死亡[8]。此外,ROS可通过诱导的内皮细胞Ca2+升高导致内皮细胞功能障碍的发生[11]。也有研究表明,ROS通过损害线粒体功能从而引起内皮细胞损伤。线粒体是ROS的重要细胞来源,ROS产生过多时释放促炎因子,可直接损害线粒体呼吸链功能,导致氧化应激损伤进一步加重[12-13]。过量ROS产生也会导致线粒体内外膜通透性改变及离子浓度不平衡,引起内皮炎症发生,从而诱导细胞凋亡和衰老[14]。
电离辐射也可通过诱发炎症反应引起血管内皮细胞损伤。研究表明电离辐射可通过激活NF-κB途径导致活化白细胞从而引起黏附和迁移的增加[15-17]。辐射引起的DNA的直接或间接损伤产生DNA双链断裂(double-strand breakage,DSBs),并进一步激活共济失调毛细血管扩张突变激酶(ataxia telangiectasia-mutated kinase,ATM),激活的ATM进一步促进IKK-γ/NF-κB基本调节剂(NF-κB essential modulator, NEMO)的核输出,从而激活细胞质中的NF-κB,并释放炎性SASP分子,如白介素-6(IL-6)、白介素-8(IL-8)、肿瘤坏死因子-α(tumor necrosis factor,TNF-α)等,最终影响内皮细胞与白细胞间相互作用,诱导内皮细胞功能障碍[18],破坏血管壁并抑制血管生成。此外,电离辐射诱导细胞损伤并释放损伤相关分子模式(damage-associated molecular patterns,DAMPs),激活全身炎症反应,导致内皮功能障碍[19]。
综上所述,电离辐射通过氧化应激和炎症反应等机制诱导血管内皮细胞损伤,损伤程度与受照剂量及时间相关。
二、衰老在辐射损伤中的作用细胞衰老(cell aging)是指细胞在执行生命活动过程中,随着时间的推移,细胞增殖分化能力和生理功能逐渐发生衰退的变化过程。一般认为,细胞衰老主要有两种类型:复制性衰老(replicative senescence)和应激诱导的过早衰老(stress-induced premature senescence,SIPS)[20]。电离辐射作为应激源可触发细胞SIPS [21]。辐射触发的内皮细胞衰老表现出多种表型,包括细胞形态改变、永久性细胞周期阻滞、衰老相关β-半乳糖苷酶(senescence-associated β-galactosidase,SA-β-gal)染色增加、溶酶体数目增加、脂褐素积累、p53及p16表达增加等,并在血管生成方面存在障碍。此外,衰老的内皮细胞可表达SASP分子,SASP主要成分包括细胞因子、胞外基质蛋白酶、生长因子等,例如IL-6、IL-8、白介素1(IL-1)、TNF-α、单核细胞趋化蛋白-1(monocyte chemotactic protein-1,MCP-1)、纤溶酶原激活物抑制因子-1(plasminogen activator inhibitor 1,PAI-1)、血管内皮生长因子(vascular endothelial growth factor,VEGF)、细胞间黏附分子(intercellular adhesion molecule,ICAM)和血管细胞黏附分子(vascular cell adhesion molecule-1,VCAM-1)等[22]。不同的诱因及不同的细胞类型会分泌不同的SASP分子[23]。p16INK4a和p53在电离辐射诱导的内皮细胞衰老过程中表达也增加[24]。因此,可通过检测细胞形态改变、细胞周期阻滞、SA-β-gal、溶酶体数量变化、脂褐素积累量、SASP、p53和p16表达检测细胞衰老的发生。
实验和流行病学数据均表明电离辐射可诱导血管内皮细胞发生DNA损伤和氧化应激反应,引起内皮细胞发生衰老,并释放早衰相关的炎性因子,衰老细胞积累会诱发慢性炎症,进一步引起内皮功能损伤,并最终导致心血管疾病的发生发展[25-30]。此外,在动物模型中电离辐射可诱导内皮细胞衰老也得到了验证,其中SASP因子表达增加,过氧化物酶体增殖物激活受体α(peroxisome proliferators-activated receptors α,PPARα)、Sirtuins蛋白(SIRT)、雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)等信号通路激活[31-33],引发炎症反应,从而引起辐射损伤。
三、SASP相关衰老在辐射所致血管内皮细胞损伤中的作用辐射诱导血管内皮细胞损伤主要涉及氧化应激和炎症,其中,辐射通过细胞周期阻滞和分泌SASP[34-36]因子引起血管内皮细胞早衰。电离辐射引起细胞早衰及损伤的已知通路见图 1。
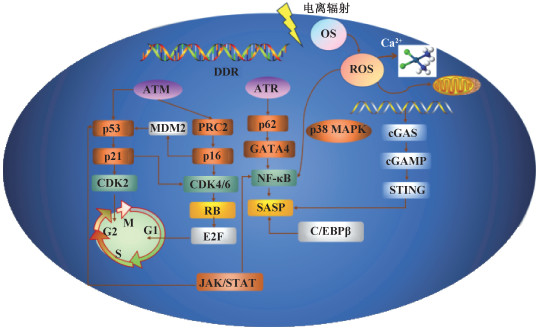
|
注:OS. 氧化应激;DDR. DNA损伤反应;ROS. 活性氧;ATM. 共济失调毛细血管扩张突变激酶;ATR. ATM和Rad3相关蛋白;CDK2. 周期素依赖性蛋白2;MDM2. 鼠双微体2蛋白;PRC2. 多梳蛋白抑制复合物2;CDK4/6. 细胞周期蛋白依赖性激酶4/6;RB. 视网膜母细胞瘤肿瘤抑制蛋白;E2F. 核转录因子E2;GATA4. 转录因子GATA结合蛋白4;NF-κB. 核转录因子κB;SASP. 衰老相关分泌表型;p38 MAPK. p38丝裂原活化蛋白激酶;cGAS. 环GMP-AMP合酶;cGAMP. 环GMP-AMP;STING. 干扰素基因刺激蛋白;C / EBPβ. CCAAT/增强子结合蛋白β 图 1 电离辐射诱导细胞早衰相关信号通路 Figure 1 Signaling pathways associated with ionizing radiation-induced cellular senescence |
1. 细胞衰老相关信号通路
(1) p53/p21通路:由电离辐射引起的DNA损伤反应(DNA damage response,DDR)受ATM、ATM和Rad3相关蛋白(ATM and Rad3 related,ATR)以及DNA依赖性蛋白激酶(DNA dependent protein kinase,DNA-PK)调控。其中,ATM可通过激活p53从而引起细胞衰老[37]。电离辐射可引起G2期阻滞的发生,激活p53导致p21上调并抑制周期素依赖性蛋白2(recombinant cyclin dependent kinase 2,CDK2)活性,维持G2期阻滞[38]。此外,p21还可通过抑制细胞周期蛋白D1(cyclinD1)-CDK4/6复合物,间接激活视网膜母细胞瘤肿瘤抑制蛋白(RB)家族以抑制E2F1的依赖性表达,从而阻断G2/M进展并导致不可逆的细胞周期G2期阻滞[39]。p21可通过p53依赖性和非依赖性途径引起细胞衰老,p21的过表达可在上调衰老基因表达的同时下调衰老细胞中的增殖基因的表达[40],从而导致细胞早衰。
(2) p16/RB通路:RB家族是细胞周期蛋白依赖性激酶(CDK)复合物的主要靶点之一,最主要的功能是结合并灭活E2F复合物,抑制E2F靶基因转录。p16是一种肿瘤抑制因子,通过与CDK4/6结合来影响细胞周期进展,并通过与MDM2(murine double minute 2)结合来调节p53的稳定性[41]。
p16INK4a在SIPS中起着关键调控作用[34]。近年研究表明,持续的DDR可激活CDK抑制剂、p16INK4a和p21WAF1/CIP1的表达,引起正常细胞早衰。p16INK4a抑制CyclinD-CDK4/6复合物的活性,p21WAF1/CIP1阻断CyclinE-CDK2活性,这两种复合物均可通过阻断RB,从而抑制E2F靶基因的表达并诱导细胞周期阻滞的发生[42]。p16的激活在衰老的维持和不可逆性中起着关键作用[23, 43]。p16/pRB途径既可单独作用,也可与p53-p21途径联合作用[44],引起细胞早衰的发生。
(3) 衰老相关分泌表型(SASP):衰老细胞分泌细胞因子、趋化因子、基质金属蛋白酶(matrix metalloproteinase,MMP)和其他可以改变局部组织环境的蛋白质,这一特征被称为SASP[45]。多种核因子和细胞质因子可触发SASP,并在多种方式和水平调控SASP。NF-κB相关促炎因子是SASP的关键成分,IL-6和IL-8是表达最多的细胞因子[46]。SASP通过其表达的炎症因子、促生长因子激活NF-κB信号通路,通过旁分泌效应影响邻近细胞中的细胞衰老[47]。
衰老细胞具有的先天免疫机制可感知损伤并激活SASP[48]。胞质DNA是诱导SASP的关键信号,由环GMP-AMP合酶(cyclic GMP-AMP synthase,cGAS)感知胞质DNA,然后产生环GMP-AMP(cGAMP),激活干扰素基因STING,该通路被认为是SASP表达的关键调节途径[49]。持续的DDR可激活几种促炎SASP因子(如IL-6、IL-8)的表达[50]。p38 MAPK/NF-κB通路独立于DDR诱导SASP表达[51]。此外,p38 MAPK也可通过增加mRNA丰度来诱导SASP的发生。Nacarelli等[52]发现,HMGA1下游的烟酰胺腺嘌呤二核苷酸(NAD+)与NADH比率的增加能将低促炎SASP转化为高促炎SASP,同时通过5′AMP激活蛋白激酶(5′ AMP activated protein kinase,AMPK)引起p53活化,激活促炎SASP的表达。
2. SASP转录调控研究:炎症相关SASP的发生受NF-κB和CCAAT/增强子结合蛋白β(CCAAT/enhancer binding protein β,CEBPβ)两种转录因子的调控。NF-κB和CEBPβ协同调控炎性SASP分子IL-1A、IL-6和IL-8等的转录,SASP分子以自分泌方式形成反馈环进一步提高NF-κB和CEBPβ活性从而增强SASP信号传导[53]。DDR还可以通过抑制转录因子GATA结合蛋白4(GATA-bindind protein 4,GATA4)的选择性自噬来诱导炎症和衰老的发生。GATA4被p62选择性自噬降解,在细胞衰老时,ATM和ATR抑制GATA4的降解,GATA4引发NF-κB的激活和SASP的启动,从而促进衰老的发生[54]。
JAK/STAT信号通路也参与调节SASP表达。JAK/STAT信号通路可调控数百个IFN刺激基因的转录,包括肿瘤抑制因子、干扰素调节因子1(interferon regulatory factor-1,IRF1)、信号转导转录激活因子1(recombinant signal transducer and activator of transcription 1,STAT1)和p53[55]。
3. SASP的表观遗传调控:衰老相关分泌表型基因表达受到表观遗传变化的调控。持续的DNA损伤引起蛋白酶体介导的G9a和GLP降解,导致H3K9me3的减少,并诱导IL-6和IL-8的产生[56]。此外,衰老细胞中积累的组蛋白变体H2AJ[57]和macroH2A1[58]也参与调控SASP的表达。
4. 其他因素对SASP的调控:近年来多种研究证明食物中含有的生物活性物质具有抗炎和抑制SASP因子表达的作用。姜黄素通过抑制NF-κB通路降低几种SASP因子如细胞因子TNF-α和IL-1表达,并增加黏附因子ICAM-1和VCAM-1表达。Matacchione等[59]发现,白藜芦醇、姜黄素和β-石竹烯混合使用能够降低部分炎症miRNA的表达,如miR-21和miR-146a,并引起参与维持内皮细胞功能的miR-126的表达升高。此外,白藜芦醇、姜黄素和β-石竹烯的混合使用与IL-1B转录水平的显著降低有关,表明混合使用生物活性物质能够抑制促炎反应和SASP表达[59]。橄榄酚类物质可维持层压蛋白B1的表达,抑制电离辐射诱导的衰老过程中cGAS/STING/NF-κB介导的SASP的产生[60]。肠道菌群产生的丁酸盐和亚精胺通过靶向NF-κB调控SASP因子如IL-8或IL-6的表达[61]。此外,有研究证明,环境有害因素如无机砷等可调控SASP的表达,引起细胞发生早衰。Okamura等[62]研究证明亚砷酸盐可引起DNA损伤,使得SASP因子mRNA和蛋白水平均升高,从而导致细胞早衰的发生。
四、挑战与展望电离辐射诱导的细胞衰老通过p53/p21和p16/RB通路的激活,导致细胞周期阻滞。此外,SASP通过促进炎症状态也可引起细胞衰老。SASP促进ICAM和VCAM-1等内皮细胞表面黏附因子表达和IL-6、IL-8、IL-1、TNF-α和TNF-β等细胞因子释放[63],影响白细胞与内皮细胞相互作用,导致中性粒细胞与内皮细胞黏附发生改变,破坏血管壁完整性和通透性。SASP相关早衰引发炎症状态诱导血管内皮细胞损伤,破坏血管壁并抑制血管生成,最终导致相关疾病如心血管疾病的发生。抑制炎症对于减少内皮衰老、阻止衰老依赖性内皮结构和功能变化以及相关的血管功能障碍,具有重要意义。
利益冲突 无
作者贡献声明 伊如罕负责论文撰写;刘萌萌、陈婕、李辰提供建议和论文修改;高玲提供研究思路和指导论文修改;刘青杰指导论文修改
| [1] |
王梦迪, 李敏, 曹璐, 等. 心血管内皮细胞放射性损伤机制的研究进展[J]. 心血管病学进展, 2021, 42(2): 101-105. Wang MD, Li M, Cao L, et al. Mechanism of radiation-induced cardiovascular endothelial cell injury[J]. Adv Cardiovasc Dis, 2021, 42(2): 101-105. DOI:10.16806/j.cnki.issn.1004-3934.2021.02.002 |
| [2] |
Sharma GP, Himburg HA. Organ-specific endothelial dysfunction following total body irradiation exposure[J]. Toxics, 2022, 10(12): 747. DOI:10.3390/toxics10120747 |
| [3] |
赵红玲, 宋曼, 关华, 等. 电离辐射诱导血管内皮细胞衰老的研究进展[J]. 国际放射医学核医学杂志, 2021, 45(6): 396-402. Zhao HL, Song M, Guan H, et al. Research progress on vascular endothelial cell senescence induced by ionizing radiation[J]. Int J Radiat Med Nucl Med, 2021, 45(6): 396-402. DOI:10.3760/cma.j.cn121381-202006045-00067 |
| [4] |
Satyamitra MM, DiCarlo AL, Taliaferro L. Understanding the pathophysiology and challenges of development of medical countermeasures for radiation-induced vascular/endothelial cell injuries: Report of a NIAID workshop, August 20, 2015[J]. Radiat Res, 2016, 186(2): 99-111. DOI:10.1667/RR14436.1 |
| [5] |
Panganiban RA, Mungunsukh O, Day RM. X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells[J]. Int J Radiat Biol, 2013, 89(8): 656-667. DOI:10.3109/09553002.2012.711502 |
| [6] |
Yentrapalli R, Azimzadeh O, Sriharshan A, et al. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation[J]. PLoS One, 2013, 8(8): e70024. DOI:10.1371/journal.pone.0070024 |
| [7] |
Yentrapalli R, Azimzadeh O, Barjaktarovic Z, et al. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation[J]. Proteomics, 2013, 13(7): 1096-1107. DOI:10.1002/pmic.201200463 |
| [8] |
Wijerathne H, Langston JC, Yang Q, et al. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies[J]. Radiother Oncol, 2021, 158: 21-32. DOI:10.1016/j.radonc.2021.02.007 |
| [9] |
Nagane M, Yasui H, Kuppusamy P, et al. DNA damage response in vascular endothelial senescence: Implication for radiation-induced cardiovascular diseases[J]. J Radiat Res, 2021, 62(4): 564-573. DOI:10.1093/jrr/rrab032 |
| [10] |
Catar R, Chen L, Zhao H, et al. Native and oxidized low-density lipoproteins increase the expression of the LDL receptor and the LOX-1 receptor, respectively, in arterial endothelial cells[J]. Cells, 2022, 11(2): 204. DOI:10.3390/cells11020204 |
| [11] |
Negri S, Faris P, Moccia F. Reactive oxygen species and endothelial CA2+ signaling: Brothers in arms or partners in crime?[J]. Int J Mol Sci, 2021, 22(18): 9821. DOI:10.3390/ijms22189821 |
| [12] |
Chang X, Zhao Z, Zhang W, et al. Natural antioxidants improve the vulnerability of cardiomyocytes and vascular endothelial cells under stress conditions: A focus on mitochondrial quality control[J]. Oxid Med Cell Longev, 2021, 2021: 6620677. DOI:10.1155/2021/6620677 |
| [13] |
Minjares M, Wu W, Wang JM. Oxidative stress and micrornas in endothelial cells under metabolic disorders[J]. Cells, 2023, 12(9): 1341. DOI:10.3390/cells12091341 |
| [14] |
Heimer S, Knoll G, Schulze-Osthoff K, et al. Raptinal bypasses BAX, BAK, and BOK for mitochondrial outer membrane permeabilization and intrinsic apoptosis[J]. Cell Death Dis, 2019, 10(8): 556. DOI:10.1038/s41419-019-1790-z |
| [15] |
Gunawardena T, Merinopoulos I, Wickramarachchi U, et al. Endothelial dysfunction and coronary vasoreactivity-a review of the history, physiology, diagnostic techniques, and clinical relevance[J]. Curr Cardiol Rev, 2021, 17(1): 85-100. DOI:10.2174/1573403X16666200618161942 |
| [16] |
Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, et al. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches[J]. Int J Mol Sci, 2021, 22(8): 3850. DOI:10.3390/ijms22083850 |
| [17] |
Xie W, Huang W, Cai S, et al. NF-κB/IκBα signaling pathways are essential for resistance to heat stress-induced ROS production in pulmonary microvascular endothelial cells[J]. Mol Med Rep, 2021, 24(5): 814. DOI:10.3892/mmr.2021.12454 |
| [18] |
Venkatesulu BP, Mahadevan LS, Aliru ML, et al. Radiation-induced endothelial vascular injury: A review of possible mechanisms[J]. JACC Basic Transl Sci, 2018, 3(4): 563-572. DOI:10.1016/j.jacbts.2018.01.014 |
| [19] |
Liu XC, Zhou PK. Tissue reactions and mechanism in cardiovascular diseases induced by radiation[J]. Int J Mol Sci, 2022, 23(23): 14786. DOI:10.3390/ijms232314786 |
| [20] |
Mikuła-Pietrasik J, Niklas A, Uruski P, et al. Mechanisms and significance of therapy-induced and spontaneous senescence of cancer cells[J]. Cell Mol Life Sci, 2020, 77(2): 213-229. DOI:10.1007/s00018-019-03261-8 |
| [21] |
Calcinotto A, Kohli J, Zagato E, et al. Cellular senescence: Aging, cancer, and injury[J]. Physiol Rev, 2019, 99(2): 1047-1078. DOI:10.1152/physrev.00020.2018 |
| [22] |
Ungvari Z, Podlutsky A, Sosnowska D, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity[J]. J Gerontol A Biol Sci Med Sci, 2013, 68(12): 1443-1457. DOI:10.1093/gerona/glt057 |
| [23] |
Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease[J]. Nat Rev Mol Cell Biol, 2020, 21(4): 183-203. DOI:10.1038/s41580-019-0199-y |
| [24] |
Yang J, Liu M, Hong D, et al. The paradoxical role of cellular senescence in cancer[J]. Front Cell Dev Biol, 2021, 9: 722205. DOI:10.3389/fcell.2021.722205 |
| [25] |
Klaus R, Niyazi M, Lange-Sperandio B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis[J]. Radiat Oncol, 2021, 16(1): 43. DOI:10.1186/s13014-021-01764-y |
| [26] |
Ramadan R, Baatout S, Aerts A, et al. The role of connexin proteins and their channels in radiation-induced atherosclerosis[J]. Cell Mol Life Sci, 2021, 78(7): 3087-3103. DOI:10.1007/s00018-020-03716-3 |
| [27] |
Azimzadeh O, Azizova T, Merl-Pham J, et al. Chronic occupational exposure to ionizing radiation induces alterations in the structure and metabolism of the heart: A proteomic analysis of human formalin-fixed paraffin-embedded (FFPE) cardiac tissue[J]. Int J Mol Sci, 2020, 21(18): 6832. DOI:10.3390/ijms21186832 |
| [28] |
Li W, Nyhan MM, Wilker EH, et al. Recent exposure to particle radioactivity and biomarkers of oxidative stress and inflammation: The Framingham heart study[J]. Environ Int, 2018, 121(Pt 2): 1210-1216. DOI:10.1016/j.envint.2018.10.039 |
| [29] |
Huang S, Garshick E, Vieira C, et al. Short-term exposures to particulate matter gamma radiation activities and biomarkers of systemic inflammation and endothelial activation in COPD patients[J]. Environ Res, 2020, 180: 108841. DOI:10.1016/j.envres.2019.108841 |
| [30] |
Blomberg AJ, Nyhan MM, Bind MA, et al. The role of ambient particle radioactivity in inflammation and endothelial function in an elderly cohort[J]. Epidemiology, 2020, 31(4): 499-508. DOI:10.1097/EDE.0000000000001197 |
| [31] |
Epperly MW, Shields D, Fisher R, et al. Radiation-induced senescence in p16+/LUC mouse lung compared to bone marrow multilineage hematopoietic progenitor cells[J]. Radiat Res, 2021, 196(3): 235-249. DOI:10.1667/RADE-20-00286.1 |
| [32] |
Azimzadeh O, Merl-Pham J, Subramanian V, et al. Late effects of chronic low dose rate total body irradiation on the heart proteome of apoe(-/-) mice resemble premature cardiac ageing[J]. Cancers (Basel), 2023, 15(13): 3417. DOI:10.3390/cancers15133417 |
| [33] |
Wang Z, Chen Z, Jiang Z, et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents[J]. Nat Commun, 2019, 10(1): 2538. DOI:10.1038/s41467-019-10386-8 |
| [34] |
Babini G, Baiocco G, Barbieri S, et al. A systems radiation biology approach to unravel the role of chronic low-dose-rate gamma-irradiation in inducing premature senescence in endothelial cells[J]. PLoS One, 2022, 17(3): e0265281. DOI:10.1371/journal.pone.0265281 |
| [35] |
Roger L, Tomas F, Gire V. Mechanisms and regulation of cellular senescence[J]. Int J Mol Sci, 2021, 22(23): 13173. DOI:10.3390/ijms222313173 |
| [36] |
López-Otín C, Kroemer G. Hallmarks of health[J]. Cell, 2021, 184(1): 33-63. DOI:10.1016/j.cell.2020.11.034 |
| [37] |
Sun X, Feinberg MW. Vascular endothelial senescence: Pathobiological insights, emerging long noncoding rna targets, challenges and therapeutic opportunities[J]. Front Physiol, 2021, 12: 693067. DOI:10.3389/fphys.2021.693067 |
| [38] |
Mijit M, Caracciolo V, Melillo A, et al. Role of p53 in the regulation of cellular senescence[J]. Biomolecules, 2020, 10(3): 420. DOI:10.3390/biom10030420 |
| [39] |
Huang W, Hickson LJ, Eirin A, et al. Cellular senescence: the good, the bad and the unknown[J]. Nat Rev Nephrol, 2022, 18(10): 611-627. DOI:10.1038/s41581-022-00601-z |
| [40] |
Kumar S, Suman S, Fornace AJ, et al. Intestinal stem cells acquire premature senescence and senescence associated secretory phenotype concurrent with persistent DNA damage after heavy ion radiation in mice[J]. Aging (Albany NY), 2019, 11(12): 4145-4158. DOI:10.18632/aging.102043 |
| [41] |
Kumari R, Jat P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype[J]. Front Cell Dev Biol, 2021, 9: 645593. DOI:10.3389/fcell.2021.645593 |
| [42] |
Liao Z, Yeo HL, Wong SW, et al. Cellular senescence: Mechanisms and therapeutic potential[J]. Biomedicines, 2021, 9(12): 1769. DOI:10.3390/biomedicines9121769 |
| [43] |
Kudlova N, De Sanctis JB, Hajduch M. Cellular senescence: Molecular targets, biomarkers, and senolytic drugs[J]. Int J Mol Sci, 2022, 23(8): 4168. DOI:10.3390/ijms23084168 |
| [44] |
Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease: from mechanisms to therapy[J]. Nat Med, 2015, 21(12): 1424-1435. DOI:10.1038/nm.4000 |
| [45] |
Lopes-Paciencia S, Saint-Germain E, Rowell MC, et al. The senescence-associated secretory phenotype and its regulation[J]. Cytokine, 2019, 117: 15-22. DOI:10.1016/j.cyto.2019.01.013 |
| [46] |
Wang QQ, Yin G, Huang JR, et al. Ionizing radiation-induced brain cell aging and the potential underlying molecular mechanisms[J]. Cells, 2021, 10(12): 3570. DOI:10.3390/cells10123570 |
| [47] |
Aratani S, Tagawa M, Nagasaka S, et al. Radiation-induced premature cellular senescence involved in glomerular diseases in rats[J]. Sci Rep, 2018, 8(1): 16812. DOI:10.1038/s41598-018-34893-8 |
| [48] |
Hari P, Millar FR, Tarrats N, et al. The innate immune sensor Toll-like receptor 2 controls the senescence-associated secretory phenotype[J]. Sci Adv, 2019, 5(6): eaaw0254. DOI:10.1126/sciadv.aaw0254 |
| [49] |
Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer[J]. Nature, 2017, 550(7676): 402-406. DOI:10.1038/nature24050 |
| [50] |
Khalil R, Diab-Assaf M, Lemaitre JM. Emerging therapeutic approaches to target the dark side of senescent cells: New hopes to treat aging as a disease and to delay age-related pathologies[J]. Cells, 2023, 12(6): 915. DOI:10.3390/cells12060915 |
| [51] |
Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype[J]. EMBO J, 2011, 30(8): 1536-1548. DOI:10.1038/emboj.2011.69 |
| [52] |
Nacarelli T, Lau L, Fukumoto T, et al. NAD(+) metabolism governs the proinflammatory senescence-associated secretome[J]. Nat Cell Biol, 2019, 21(3): 397-407. DOI:10.1038/s41556-019-0287-4 |
| [53] |
Muromoto R, Sato A, Komori Y, et al. Regulation of NFKBIZ gene promoter activity by STAT3, C/EBPβ, and STAT1[J]. Biochem Biophys Res Commun, 2022, 613: 61-66. DOI:10.1016/j.bbrc.2022.04.140 |
| [54] |
Kang C, Xu Q, Martin TD, et al. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4[J]. Science, 2015, 349(6255): aaa5612. DOI:10.1126/science.aaa5612 |
| [55] |
Hu Q, Bian Q, Rong D, et al. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens[J]. Front Bioeng Biotechnol, 2023, 11: 1110765. DOI:10.3389/fbioe.2023.1110765 |
| [56] |
Abd Al-Razaq MA, Freyter BM, Isermann A, et al. Role of histone variant H2A.J in fine-tuning chromatin organization for the establishment of ionizing radiation-induced senescence[J]. Cells, 2023, 12(6): 916. DOI:10.3390/cells12060916 |
| [57] |
Starkova T, Polyanichko A, Tomilin AN, et al. Structure and functions of HMGB2 protein[J]. Int J Mol Sci, 2023, 24(9): 8334. DOI:10.3390/ijms24098334 |
| [58] |
Han X, Lei Q, Xie J, et al. Potential regulators of the senescence-associated secretory phenotype during senescence and aging[J]. J Gerontol A Biol Sci Med Sci, 2022, 77(11): 2207-2218. DOI:10.1093/gerona/glac097 |
| [59] |
Matacchione G, Gurǎu F, Silvestrini A, et al. Anti-SASP and anti-inflammatory activity of resveratrol, curcumin and β-caryophyllene association on human endothelial and monocytic cells[J]. Biogerontology, 2021, 22(3): 297-313. DOI:10.1007/s10522-021-09915-0 |
| [60] |
Frediani E, Scavone F, Laurenzana A, et al. Olive phenols preserve lamin B1 expression reducing cGAS/STING/NFκB-mediated SASP in ionizing radiation-induced senescence[J]. J Cell Mol Med, 2022, 26(8): 2337-2350. DOI:10.1111/jcmm.17255 |
| [61] |
Lilja S, Oldenburg J, Pointner A, et al. Epigallocatechin gallate effectively affects senescence and anti-SASP via sirt3 in 3t3-l1 preadipocytes in comparison with other bioactive substances[J]. Oxid Med Cell Longev, 2020, 2020: 4793125. DOI:10.1155/2020/4793125 |
| [62] |
Okamura K, Sato M, Suzuki T, et al. Inorganic arsenic exposure-induced premature senescence and senescence-associated secretory phenotype (SASP) in human hepatic stellate cells[J]. Toxicol Appl Pharmacol, 2022, 454: 116231. DOI:10.1016/j.taap.2022.116231 |
| [63] |
Baselet B, Belmans N, Coninx E, et al. Functional gene analysis reveals cell cycle changes and inflammation in endothelial cells irradiated with a single X-ray dose[J]. Front Pharmacol, 2017, 8: 213. DOI:10.3389/fphar.2017.00213 |
 2024, Vol. 44
2024, Vol. 44


