2. 蚌埠医科大学第二附属医院放疗科, 蚌埠 233000;
3. 皖南医学院第二附属医院肿瘤放疗科, 芜湖 241000
2. Department of Radiation Oncology, Second Affiliated Hospital of Bengbu Medical University, Bengbu 233000, China;
3. Second Affiliated Hospital of Wannan Medical College, Wuhu 241000, China
小细胞肺癌(small cell lung cancer, SCLC)是种具有高侵袭性的肺癌亚型,占所有肺癌的13%~20%,易向远处转移,50%~80%的患者在治疗过程中即发展为脑转移,从而导致生活质量下降,生存时间缩短[1-3]。既往研究指出,单纯手术及化疗对于脑转移瘤的治疗效果不佳[4-6]。随着放射治疗的兴起,全脑放疗(whole brain radiation therapy,WBRT)广泛应用于脑转移瘤的治疗,改善了患者的神经系统症状,但是局部复发率较高,仍限制着患者的局部控制情况[7]。既往研究表明,在脑转移数目较少的患者中,全脑联合局部加量放疗模式(WBRT + boost)表现出更好的局控能力及生存潜力[8-9]。主要模式有同步加量(simultaneous integrated boost,SIB)和序贯加量(sequential integrated boost, SEB),而其在小细胞肺癌脑转移方面尚无关于明确生存获益以及加量时序的定论。本研究拟分析小细胞肺癌有限个数(≤10个)脑转移患者不同颅内放疗模式下的生存差异,探索患者的预后影响因素以及最佳的颅内放疗模式。
资料与方法1. 病例资料:回顾性收集2019年1月至2022年12月就诊于蚌埠医科大学第一附属医院放疗中心接受放疗的小细胞肺癌脑转移(SCLC-BM)患者资料。纳入标准:①经组织病理学证实的SCLC。②影像学资料(CT、MRI或PET-CT)证实发生脑转移。可测量的脑转移数目≤10个(根据RESIST v1.1标准)。③KPS评分≥60,或单纯由颅内肿瘤所致KPS≤50。④没有威胁生命的急慢性基础疾病,如严重的自身免疫疾病、严重的心肺功能不全、肝肾功能不全或严重贫血等。⑤预计生存期超过3个月。排除标准:①脑转移数目>10个的患者。②放疗计划未完成或计划放疗剂量未达到的患者。③发生颅骨转移或脑膜转移的患者。④既往接受脑部放射治疗或接受脑转移瘤切除术的患者。⑤合并肺癌以外其他类型癌症的患者。所有患者均签署知情同意书,该研究由蚌埠医科大学第一附属医院伦理委员会审核批准(伦理编号:2023YJS150)。
2. 治疗及辅助治疗:患者采用调强放射治疗(Intensity modulated radiotherapy, IMRT)、容积调强放疗(Volumetric Modulated Arc Therapy, VMAT)或螺旋断层放射治疗系统(TOMO therapy system)等放疗技术,6~10 MV X射线。靶区描述:WBRT组总剂量在30~40 Gy,2~3 Gy/次,最常见方案30 Gy/10次和40 Gy/20次,中位生物有效剂量(biological effective dose, BED)为39 Gy。根据公式BED= nd × [1+d/(α/β)][10]计算,其中,脑组织的α/β设定为10,n为治疗次数,d为单次剂量。加量组局部肿瘤累计总剂量在40~60 Gy, WBRT+SIB组中位BED为62.50 Gy; WBRT+SEB组中位BED为66.30 Gy。保护常规危及器官(OARs),包括晶状体、眼睛、视神经、视交叉、垂体、脑干和脊髓。放疗期间根据患者情况及医师的临床经验,患者接受不同剂量的甘露醇、甘油果糖及激素脱水降颅压处理,部分患者接受了全身治疗,包括化疗、靶向治疗及免疫治疗。
3. 随访及评价:按期复查和随访。总生存期(OS)定义为自放疗开始至因任何原因导致死亡。颅内无进展生存期(IPFS)定义为自放疗开始至颅内发生进展或因任何原因死亡。治疗相关不良反应使用美国放射治疗协作组织(Radiation Therapy Oncology Group,RTOG)急性放射反应的评价标准,评价中枢神经系统及血液学不良反应,分为0~4级,共5级。末次随访时间为2023年11月30日。
4. 统计学处理:数据采用SPSS软件27.0版本进行统计分析,Graphpad prism 9软件绘制生存曲线。组间计数资料采用χ2或Fisher精确检验,等级资料采用秩和检验。生存分析采用Kaplan-Meier法,并采用log-rank检验组间差异及单因素分析。单因素分析中P<0.1以及可能具有临床意义的变量纳入多因素Cox回归分析。P<0.05为差异有统计学意义。
结果1. 临床资料:本研究共纳入143例符合标准的患者,WBRT组58例、WBRT+SIB组53例,WBRT+SEB组32例患者。患者基线特征及治疗情况见表 1。其中男性114例(79.7%)、女性29例(20.3%),平均年龄(62.2±8.5)岁,中位年龄63岁。3组基线资料比较差异无统计学意义(P>0.05)。
|
|
表 1 小细胞肺癌脑转移患者的基线特征 Table 1 Baseline characteristics of patients with brain metastases from small cell lung cancer |
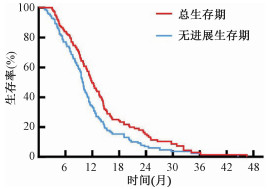
2. 生存情况:截至随访日,全组患者中位OS、中位IPFS分别为11.9、9.9个月;1、2、3年生存率分别为49.7%、15.3%、2.9%,见图 1。
|
图 1 143例肺癌脑转移患者的总生存(OS)和无进展生存(IPFS)曲线 Figure 1 Overall survival(OS) and intracranial progression-free survival(IPFS)curves of 143 patients with brain metastases from small cell lung cancer |
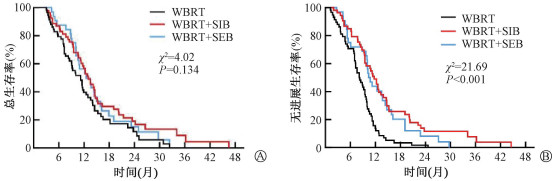
WBRT组、WBRT+SIB组及WBRT+SEB组患者中位OS分别为11.2、13.0、12.5个月,差异无统计学意义(P>0.05),组间比较差异无统计学意义(P>0.05),见图 2A。3组中位IPFS分别为8.1、11.7、10.4个月,差异有统计学意义(χ2=21.69,P<0.001)见图 2B;行组间比较显示,WBRT+SIB组及WBRT+SEB组相比于WBRT组,差异均有统计学意义(WBRT+SIB vs. WBRT, χ2=18.13, P<0.001; WBRT+SEB vs. WBRT, χ2=10.28, P=0.001),而同步加量组与序贯加量组相比,差异无统计学意义(P>0.05)。
|
注:WBRT.全脑放疗组; WBRT+SIB.全脑联合同步加量放疗组;WBRT+SEB.全脑联合序贯加量放疗组 图 2 肺癌脑转移患者不同颅内放疗模式患者的生存曲线 A. 总生存(OS);B.颅内无进展生存(IPFS) Figure 2 Survival curves of patients with brain metastases from small cell lung cancer under different intracranial radiotherapy modalities A. Overall survival (OS); B. Intracranial progression-free survival (IPFS) |
由于本样本中少发脑转移(≤3个)患者例数较多(90例),对其进行单独分析,WBRT组、WBRT+SIB组及WBRT+SEB组中位OS分别为11.5、14.4、13.7个月,差异有统计学意义(χ2=8.72,P=0.013),见图 3A,且WBRT+SIB组及WBRT+SEB组优于WBRT组(WBRT+SIB vs. WBRT, χ2=7.32, P=0.007; WBRT+SEB vs. WBRT, χ2=3.94, P=0.047)。三组中位IPFS为8.9、12.6、10.4个月,差异有统计学意义(χ2=12.37,P=0.002),见图 3B,且WBRT+SIB组及WBRT+SEB组优于WBRT组(WBRT+SIB vs. WBRT, χ2=11.32, P<0.001; WBRT+SEB vs. WBRT, χ2=4.61, P=0.032)。而WBRT+SIB组与WBRT+SEB组在OS及IPFS方面相比,差异均无统计学意义(P>0.05)。

|
注:WBRT.全脑放疗组; WBRT+SIB.全脑联合同步加量放疗组;WBRT+SEB.全脑联合序贯加量放疗组 图 3 肺癌≤3个脑转移灶的不同颅内放疗模式患者的生存曲线 A.总生存(OS);B. 无进展生存(IPFS) Figure 3 Survival curves of three groups of patients with ≤3 brain metastases from small cell lung cancer under different intracranial radiotherapy modalities A. Overall survival (OS); B. Intracranial progression-free survival (IPFS) |
3. 治疗相关不良反应:主要对放疗所致中枢神经系统及血液系统不良反应进行分析,治疗中,中枢神经系统急性反应达到2级及以上的WBRT组有16例(27.6%),WBRT+SIB组有14例(26.4%),WBRT+SEB组有9例(28.1%),差异无统计学意义(P>0.05),未发现4级的急性放射反应。血液学反应达到2级及以上的WBRT组有10例(17.2%),WBRT+SIB组有9例(17.0%),WBRT+SEB组有7例(21.9%),差异无统计学意义(P>0.05)。
4. 预后分析:单因素分析提示年龄、脑转移数目、KPS评分、淋巴结转移、体质量指数(BMI)水平、后续化疗、靶向及免疫治疗与OS相关。年龄、脑转移数目、淋巴结转移、BMI水平、放疗模式、后续化疗、靶向及免疫治疗与IPFS有关,见表 2。多因素分析提示BMI水平、后续化疗、靶向及免疫治疗是患者OS的独立预后因素(HR=0.63、0.42、0.58、0.40,P<0.05),脑转移数目、淋巴结转移、后续化疗、靶向及免疫治疗以及WBRT+SIB或WBRT+SEB是影响患者IPFS的独立预后因素(HR=0.66、1.84、0.42、0.67、0.41、0.40、0.58,P<0.05),见表 3。
|
|
表 2 143例肺癌脑转移患者生存预后的单因素分析 Table 2 Univariate analysis of survival and prognosis of 143 patients with brain metastases from small cell lung cancer |
|
|
表 3 143例肺癌脑转移患者生存预后的多因素分析 Table 3 Multivariate analysis of survival and progresis of 143 patients with brain metastases of small cell lung cancer |
讨论
目前小细胞肺癌患者治疗手段多样,在WBRT的介入下,生存期较前延长,颅内转移瘤控制情况或成为限制患者放疗后存活时间的重要因素[11]。有研究对WBRT + boost模式在SCLC-BM患者中的应用展开探索,Sun等[12]对82例SCLC-BM患者的研究表明,接受WBRT + boost的患者生存时间长于单纯WBRT(13.4 vs. 8.5个月, P = 0.004), 在1~3个脑转移瘤的患者中,WBRT + boost的OS也比单独WBRT更长(13.4 vs. 9.6个月, P = 0.022)。为减小脑转移数目差异对患者生存时间的影响,本研究排除了脑转移个数>10个的病例,全部患者脑转移数目在组间差异无统计学意义,接受WBRT+SIB或WBRT+SEB模式仅在IPFS方面展现出优势。而在1~3个脑转移中,WBRT+SIB及WBRT+SEB模式展现出OS及IPFS的双重获益。Ni等[13]配对比较WBRT、SRS及WBRT + boost 3种治疗手段的SCLC-BM患者,发现WBRT + boost在OS及IPFS的表现优于另两组。而本研究中心因使用SRS仅6例,未将其纳入研究。多项回顾性研究表明,全脑联合局部放疗时,高于常规的生物有效剂量(BED)可有效改善患者的生存率及颅内无进展生存时间[14-15]。但是上述研究均未比较局部加量放疗时序对患者的影响。本研究中同步加量放疗与序贯加量放疗相比无明显差异。
既往认为年龄、脑转移个数、KPS评分、脑转移发生时间、放疗模式及放疗后的全身治疗等可能是影响脑转移患者预后的影响因素[15-16]。本研究多因素分析显示,BMI水平、放疗后续的化疗、靶向治疗及免疫治疗是影响患者OS的独立危险因素。既往有研究表明BMI与肺癌死亡风险呈负相关[17]。Jiang等[18]研究肯定了超重和肥胖(BMI≥25 kg/m2)在接受以铂为基础化疗的ES-SCLC患者预后中的潜力。同样,本研究中高水平BMI(≥25 kg/m2)提示患者预后较好。
有研究认为,放射线会通过促进神经炎症反应,诱导内皮细胞凋亡、抑制血管内皮细胞增殖等方面改变血脑屏障(BBB)及血-肿瘤屏障(BTB)的通透性[19-20]。一些小分子化疗药物和酪氨酸激酶抑制剂等在此情形下有望提高转移瘤区域的药物浓度,为患者带来获益[21-22]。与使用WBRT相比,联合治疗时患者Ⅲ/Ⅳ级血液学和胃肠道不良反应的风险增加[23]。近年来,免疫检查点抑制剂(ICIs)在小细胞肺癌中的研究多集中于PD-1及PD-L1[24-25]。Wu等[26]发现,与其他转移器官相比,脑部放疗带来的免疫激活效果最强。由于BTB的通透性增加及射线的协同作用,ICIs可以进入大脑,破坏由肿瘤细胞引起的免疫抑制微环境,通过抑制检查点与其配体的相互作用来恢复CTL的毒性作用从而杀死肿瘤细胞,然而联合使用的最佳时序仍未确定[27]。适时联合全身治疗有望维持颅内控制及长期生存,但是全身治疗介入时机及如何控制潜在增加的不良反应需要进一步探索。本研究中化疗药物主要包括依托泊苷、铂类、替莫唑胺等;靶向药物主要为安罗替尼;免疫治疗药物主要为PD-1/PD-L1抑制剂。结果提示,放疗期间同步全身治疗对患者预后无明显影响,放疗后足周期的化疗、靶向治疗及免疫治疗是患者预后的独立影响因素,而放疗期间同步药物治疗未带来明显获益,对指导患者后续治疗提供借鉴意义。
综上所述,在有限个数(≤10个)脑转移的SCLC患者中,BMI水平、放疗后的化疗、靶向治疗及免疫治疗是患者预后的独立影响因素,放疗模式的选择可影响患者颅内无进展生存期。WBRT联合同步加量放疗及WBRT联合序贯加量放疗模式均可进一步提高患者的IPFS。在少发脑转移(≤3个)患者中,局部加量模式为患者带来OS及IPFS双重获益,其可能成为小细胞肺癌脑转移治疗管理的新方向。
利益冲突 无
作者贡献声明 常芳芳、夏晓东负责数据整理、统计分析、论文撰写;崔珍、江浩提出研究思路、设计研究方案及论文修改;郭子文、李梦妮、刘佳收集数据与随访
| [1] |
Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2017, 18(5): 663-671. DOI:10.1016/S1470-2045(17)30230-9 |
| [2] |
Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality[J]. N Engl J Med, 2020, 383(7): 640-649. DOI:10.1056/NEJMoa1916623 |
| [3] |
Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease[J]. Neuro Oncol, 2021, 23(9): 1447-1456. DOI:10.1093/neuonc/noab101 |
| [4] |
Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study[J]. J Clin Oncol, 2011, 29(2): 134-141. DOI:10.1200/JCO.2010.30.1655 |
| [5] |
Bender E. Getting cancer drugs into the brain[J]. Nature, 2018, 561(7724): S46-S47. DOI:10.1038/d41586-018-06707-4 |
| [6] |
Seute T, Leffers P, Wilmink JT, et al. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy[J]. J Clin Oncol, 2006, 24(13): 2079-2083. DOI:10.1200/JCO.2005.03.2946 |
| [7] |
Yomo S, Hayashi M. Is stereotactic radiosurgery a rational treatment option for brain metastases from small cell lung cancer? A retrospective analysis of 70 consecutive patients[J]. BMC Cancer, 2015, 15: 95. DOI:10.1186/s12885-015-1103-6 |
| [8] |
Dobi Á, Fodor E, Maráz A, et al. Boost irradiation integrated to whole brain radiotherapy in the management of brain metastases[J]. Pathol Oncol Res, 2020, 26(1): 149-157. DOI:10.1007/s12253-018-0383-y |
| [9] |
Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial[J]. JAMA Oncol, 2015, 1(4): 457-464. DOI:10.1001/jamaoncol.2015.1145 |
| [10] |
Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy[J]. Br J Radiol, 1989, 62(740): 679-694. DOI:10.1259/0007-1285-62-740-679 |
| [11] |
马金涛, 贾惠钧, 孟春柳, 等. 全脑放疗对广泛期小细胞肺癌脑转移的生存影响[J]. 中华放射肿瘤学杂志, 2022, 31(10): 891-896. Ma JT, Jia HJ, Meng CL, et al. The impact of whole brain radiation therapy on overall survival in patients with extensive stage small cell lung cancer with brain metastases[J]. Chin J Radiat Oncol, 2022, 31(10): 891-896. DOI:10.3760/cma.j.cn113030-20220321-00110 |
| [12] |
Sun H, Xu L, Wang Y, et al. Additional radiation boost to whole brain radiation therapy may improve the survival of patients with brain metastases in small cell lung cancer[J]. Radiat Oncol, 2018, 13(1): 250. DOI:10.1186/s13014-018-1198-4 |
| [13] |
Ni M, Jiang A, Liu W, et al. Whole brain radiation therapy plus focal boost may be a suitable strategy for brain metastases in SCLC patients: a multi-center study[J]. Radiat Oncol, 2020, 15(1): 70. DOI:10.1186/s13014-020-01509-3 |
| [14] |
Zhuang QY, Li JL, Lin FF, et al. High biologically effective dose radiotherapy for brain metastases may improve survival and decrease risk for local relapse among patients with small-cell lung cancer: a propensity-matching analysis[J]. Cancer Control, 2020, 27(2): 1073274820936287. DOI:10.1177/1073274820936287 |
| [15] |
Li H, Li W, Qi C, et al. Optimizing whole brain radiotherapy treatment and dose for patients with brain metastases from small cell lung cancer[J]. Front Oncol, 2021, 11: 726613. DOI:10.3389/fonc.2021.726613 |
| [16] |
Li H, Xue R, Yang X, et al. Best supportive care versus whole-brain irradiation, chemotherapy alone, or WBRT plus chemotherapy in patients with brain metastases from small-cell lung cancer: a case-controlled analysis[J]. Front Oncol, 2021, 11: 568568. DOI:10.3389/fonc.2021.568568 |
| [17] |
Shepshelovich D, Xu W, Lu L, et al. Body mass index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the international lung cancer consortium[J]. J Thorac Oncol, 2019, 14(9): 1594-1607. DOI:10.1016/j.jtho.2019.05.031 |
| [18] |
Jiang S, Huang L, Zhen H, et al. Carboplatin versus cisplatin in combination with etoposide in the first-line treatment of small cell lung cancer: a pooled analysis[J]. BMC Cancer, 2021, 21(1): 1308. DOI:10.1186/s12885-021-09034-6 |
| [19] |
Parente A, de Vries E, van Waarde A, et al. The acute and early effects of whole-brain irradiation on glial activation, brain metabolism, and behavior: a positron emission tomography study[J]. Mol Imaging Biol, 2020, 22(4): 1012-1020. DOI:10.1007/s11307-020-01483-y |
| [20] |
Warrington JP, Ashpole N, Csiszar A, et al. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications[J]. J Vasc Res, 2013, 50(6): 445-457. DOI:10.1159/000354227 |
| [21] |
Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs[J]. Cell, 1994, 77(4): 491-502. DOI:10.1016/0092-8674(94)90212-7 |
| [22] |
Parsai S, Miller JA, Juloori A, et al. Stereotactic radiosurgery with concurrent lapatinib is associated with improved local control for HER2-positive breast cancer brain metastases[J]. J Neurosurg, 2020, 132(2): 503-511. DOI:10.3171/2018.10.JNS182340 |
| [23] |
Han J, Qiu M, Su L, et al. Response and safety of whole-brain radiotherapy plus temozolomide for patients with brain metastases of non-small-cell lung cancer: A meta-analysis[J]. Thorac Cancer, 2021, 12(23): 3177-3183. DOI:10.1111/1759-7714.14183 |
| [24] |
Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN[J]. ESMO Open, 2022, 7(2): 100408. DOI:10.1016/j.esmoop.2022.100408 |
| [25] |
Chen Y, Paz-Ares L, Reinmuth N, et al. Impact of brain metastases on treatment patterns and outcomes with first-line durvalumab plus platinum-etoposide in extensive-stage SCLC (CASPIAN): a brief report[J]. JTO Clin Res Rep, 2022, 3(6): 100330. DOI:10.1016/j.jtocrr.2022.100330 |
| [26] |
Wu M, Wu S, Chen Y, et al. Immune activation effects at different irradiated sites and optimal timing of radioimmunotherapy in patients with extensive-stage small cell lung cancer: a real-world analysis[J]. Biol Proced Online, 2023, 25(1): 24. DOI:10.1186/s12575-023-00217-y |
| [27] |
Tian W, Chu X, Tanzhu G, et al. Optimal timing and sequence of combining stereotactic radiosurgery with immune checkpoint inhibitors in treating brain metastases: clinical evidence and mechanistic basis[J]. J Transl Med, 2023, 21(1): 244. DOI:10.1186/s12967-023-04089-4 |
 2024, Vol. 44
2024, Vol. 44


