2. 军事科学院军事医学研究院辐射医学研究所 放射生物学北京市重点实验室, 北京 100850
2. Beijing Key Laboratory for Radiobiology, Beijing Institute of Radiation Medicine, Academy of Military Medical Sciences, Academy of Military Sciences, Beijing 100850, China
放射性肺损伤(radiation-induced lung injury, RILI)是核辐射事故、骨髓移植预处理及胸部肿瘤放疗后常见的并发症,可分为早期的放射性肺炎(radiation pneumonitis, RP)和后期的放射性肺纤维化(radiation-induced pulmonary fibrosis, RIPF)两个阶段[1]。RIPF以进行性和不可逆性肺部结构破坏为特征,临床表现包括进行性呼吸困难,并发心肺功能衰竭甚至死亡,其发病机制尚未阐明,目前亦无有效的治疗措施[2]。近年研究表明,电离辐射诱导的肺上皮间质转化(epithelial-mesenchymal transition, EMT)是RIPF发生发展的重要环节,本课题组研究也证明非编码RNA调控肺EMT在RIPF的发生发展中起重要作用,并可能成为RIPF的潜在治疗靶点[3-6]。本文对电离辐射诱导肺EMT在RIPF发生发展中的作用及以EMT为潜在治疗靶点的相关药物做一综述。
一、电离辐射诱导肺EMT的发生EMT是上皮细胞转化为间充质细胞的细胞表型转化过程,具有可逆性,该过程通过破坏上皮细胞间接触,使得上皮细胞失去顶端基底极性,获得运动和侵袭能力。目前公认的EMT发生标志是E-钙黏蛋白(E-cadherin)和胞质紧密黏连蛋白-1 (zonula occludens-1, ZO-1)等上皮标志物的表达减少,以及N-钙黏蛋白(N-cadherin)、波形蛋白(vimentin)和α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)等间质标志物的表达增加[7]。根据其发生的生物学背景可将EMT分为3种不同亚型[8]:Ⅰ型与植入、胚胎形成和器官发育相关。原始上皮通过EMT产生原代间充质,该原代间充质可以通过间质上皮转化(MET)重新诱导形成继发上皮,这样的次生上皮再分化产生结缔组织细胞形成组织。Ⅲ型与肿瘤进展和转移有关。与许多器官相关的次生上皮细胞转化为肿瘤细胞,恶性转化的细胞通过EMT引起侵袭和转移。而Ⅱ型与伤口愈合、组织再生和器官纤维化相关。在炎症和损伤环境中,该类型可产生成纤维细胞或其他相关细胞,以便重建组织。当炎症消失或减少,EMT的停止,有助于伤口愈合、组织再生。当炎症持续时,EMT也会持续存在,促使器官纤维化[9-10]。
与肿瘤相关EMT发生不同的是,Ⅱ型EMT主要由博莱霉素及电离辐射等外在刺激因素触发[11-12]。越来越多的小鼠和细胞证据表明,电离辐射诱发的肺EMT可促进RIPF[13-17]。RIPF是一种进行性不可逆的慢性肺部疾病,以肺泡上皮细胞的损伤、成纤维细胞过度增殖活化和细胞外基质大量沉积(extracellular matrix,ECM)为特征,并导致患者严重的生理异常和慢性呼吸衰竭[18]。成纤维细胞的异常增殖与激活是促进肺纤维化发生发展的关键因素。研究发现,在肺纤维化中30%~50%的小鼠肺成纤维细胞具有上皮细胞标志,这表明发生EMT的受损上皮细胞是成纤维细胞的重要来源[19]。
众所周知,电离辐射产生的生物效应包括直接效应和间接效应[20]。电离辐射可以通过直接作用损伤DNA、脂质、蛋白质等重要大分子,也能在细胞内通过水辐解后产生活性氧(ROS),间接导致大分子的氧化损伤,最终共同刺激驻留细胞应激反应、细胞衰老或细胞死亡等细胞毒性[21]。电离辐射导致的细胞毒性作用会引发急性损伤反应,释放损伤相关分子模式分子(DAMP),产生一个促炎和促血管生成的微环境[22]。肺组织先天免疫系统迅速响应并招募免疫效应细胞,可能在受辐射和未受辐射的肺区域导致明显的间质性肺炎[23-24]。在炎症环境下,肺间质中相关信号分子通过诱发肺上皮细胞EMT并产生大量细胞外基质,进行受损肺组织重塑,以抵抗电离辐射损伤。但与此同时,肺上皮细胞也可能发生EMT过度,导致成纤维细胞的异常增加并沉积,从而增加了肺组织纤维化的风险[25-27]。因此,有必要探索电离辐射诱导肺上皮细胞发生EMT的具体分子机制,有效阻断受损肺组织向纤维化进展。
二、电离辐射诱导肺EMT相关分子机制1. 电离辐射诱导肺EMT相关信号通路:电离辐射诱导的肺EMT的发生涉及多因子、多信号通路共同作用(图 1)。转录因子Snail、Slug、Twist和ZEB等通过直接与E-cadherin启动子区域的E-box基序(CAGGTG)结合参与调控其基因表达,被认为是触发EMT的关键因子,并协同多信号通路调控EMT过程[28]。
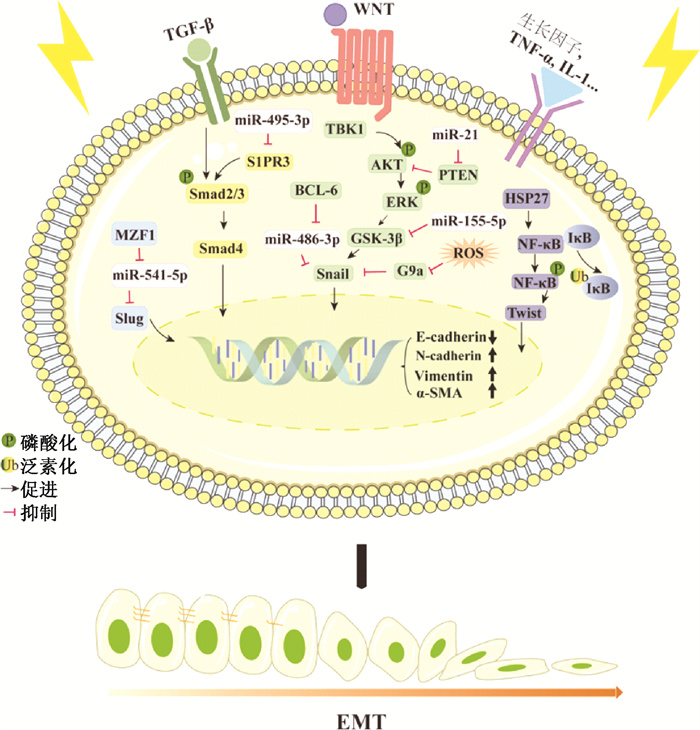
|
图 1 放射性肺纤维化中的EMT调控机制 Figure 1 Regulatory mechanism of EMT in radiation pulmonary fibrosis |
(1) 转化生长因子β(TGF-β)/Smad通路:TGF-β/Smad通路是辐射引起EMT最重要的途径,多种干扰该信号通路的途径在肺纤维化的临床前模型中显示了保护作用[29]。TGF-β可通过细胞膜上的TGF-β受体诱导Smad2和Smad3的磷酸化,并与Smad4形成复合物,激活的Smad蛋白复合物进入细胞核调节靶基因表达[30]。在最近的研究中,Zhao等[31]发现鞘氨醇-1磷酸酯受体3(sphingosine-1 phosphate receptor 3,S1PR3)可通过TGF-β/Smad通路介导肺上皮细胞的EMT过程。随后Chang等[32]和Wang等[33]发现S1PRs促进Smad蛋白磷酸化和核转运,最终导致正常组织中出现纤维化特征(ECM异常累积)。2020年Gong等[34]报道S1PR3缺陷能够抑制辐射诱导的肺上皮细胞EMT,减轻放射性肺纤维化,提示S1PR3可作为放射性肺纤维化的潜在治疗靶点。
(2) ERK通路:ERK与JNK、p38 MAPK、ERK5/MEK5同属于丝裂原活化蛋白激酶家族(mitogen-activated protein kinases,MAPKs) [35]。Nagarajan等[36]发现,辐射能特异性诱导大鼠肺Ⅱ型上皮细胞中MAPKs家族的ERK1/2发生磷酸化,并通过GSK3β/Snail促进EMT过程。而敲低ERK或使用MEK(ERK激活的上游分子)的抑制剂可逆转辐射诱发的EMT。这表明ERK1/2的磷酸化对辐射诱导肺EMT至关重要。Qu等[17]发现TANK结合激酶1(TANK-binding kinase 1,TBK1) 的缺失可有效抑制辐射诱导的EMT,缓解肺组织纤维化和胶原沉积。而进一步的研究表明,辐射诱导细胞中TBK1以及AKT和ERK的磷酸化活性以时间和剂量依赖的方式升高,且TBK1作为AKT/ERK的上游刺激因子发挥作用。以上结果表明,TBK1是肿瘤放疗期间预防放射性肺纤维化的潜在靶点。
(3) NF-κB通路:在静息状态下,NF-κB1/2与IKB激酶以异源三聚体形式稳定存在,当细胞受到外界刺激,使IKB激酶从异源三聚体中解离,被泛素-蛋白酶体系统识别并降解。NF-κB二聚体得到释放并经翻译后修饰激活,转运至细胞核中调节靶基因的转录[37]。Kang等[38-39]发现辐射能够刺激人肺上皮细胞A549中NF-κB活化,反向激活受体相互作用蛋白1(RIP1),促进STAT3和白介素1β(IL-1β)的表达与释放,该研究结果支持NF-κB参与调控辐射诱导的肺EMT过程。此外,Kim等[40]发现,在放射性肺纤维化中,热休克蛋白HSP27表达增加,使用小分子抑制其功能可以改善放射性肺纤维化。从机制上,HSP27通过与IkB激酶复合体直接相互作用激活NF-κB信号,促进EMT相关蛋白Twist、IL-6及IL-1β的表达。
2. 电离辐射诱导肺EMT相关的表观遗传调控:表观遗传修饰可以在不改变核苷酸序列的情况下,改变特定基因的转录或翻译水平,引起特定信号通路的激活或抑制,从而改变细胞生物学功能和细胞表型。据报道EMT这种细胞可塑性表型可以通过表观遗传机制记忆并传递给子细胞,其表观遗传调控机制主要包括微小RNA(miRNA)与下游RNA的结合、启动子区CpG岛甲基化水平的改变以及组蛋白修饰的多样化。
miRNA是一类小的非编码RNA(17~24个核苷酸),通过与mRNA的3'-UTR区域结合来介导基因的转录后沉默[41]。目前,研究认为有一类放射相关miRNAs参与调控电离辐射诱导的肺EMT和RIPF过程。Gong等[34]使用miR-495-3p模拟物能够抑制S1PR3蛋白的表达,有效阻断放射性肺纤维化过程中的EMT。Liu等[3]发现miR-21通过靶向PTEN/AKT通路成为电离辐射诱导的肺EMT的关键调节因子。Wang等[4]发现miR-155-5p通过靶向GSK-3β抑制电离辐射诱导的肺EMT,从而缓解RIPF。最新的研究验证了miR-541-5p和miR-486-3p参与调控放射性肺纤维化中的EMT过程,并进一步发现放射通过激活miR-541-5p和miR-486-3p的上游转录因子的表达发挥作用[5-6]。尽管如此,放射是如何诱导miRNAs表达进而参与EMT调控的机制还有待阐明。
DNA甲基化是指在基因启动子区的CpG二核苷酸的胞嘧啶上添加一个甲基基团,由DNA甲基转移酶(DNMTs) 介导[42]。E-cadherin的表达下调是细胞发生EMT最重要的标志,而其启动子区的DNA甲基化在介导其沉默中起关键作用[43-44]。Wu等[45]和Fukagawa等[46]发现,转录因子Snail与ZEB1可以募集DNMT1结合到E-cadherin的启动子CpG岛介导其沉默。Galván等[47]发现,Twist1和Twist2在结直肠癌中的高表达由其启动子甲基化水平调控。这些结果表明,Snail、ZEB和Twist等转录因子可以通过表观遗传学机制直接或间接参与E-cadherin的转录调控,从而诱导EMT。
Dong等[48]发现,H3K9me2的增加和H3K9Ac的减少与Snail诱导的时间、EMT的形态学变化以及E-cadherin启动子的DNA甲基化有关。这表明H3K9的甲基化和乙酰化修饰在沉默E-cadherin的表达中起关键作用,其中H3K9甲基化与基因表达抑制相关,而乙酰化与基因激活相关[49]。组蛋白修饰涉及一系列功能蛋白酶,其中组蛋白甲基转移酶G9a可通过在染色质中富集H3K9的单甲基化和二甲基化介导基因沉默[50]。Nagaraja等[51]在辐照处理后的肺上皮细胞中发现H3K9甲基化水平升高和EMT相关表型改变,而这些变化由辐照产生的ROS通过G9a/Snail复合物介导。结合体内研究证据,G9a可能通过介导组蛋白修饰参与放射性肺纤维化中EMT的调控[52]。
三、电离辐射诱导肺EMT靶向治疗随着电离辐射诱导肺EMT发生发展过程的逐步解析,靶向药物的研发需要紧随其后。结合现有研究数据,根据不同作用机制,总结归纳了用于放射性肺纤维化的潜在治疗药物和策略(图 2)。
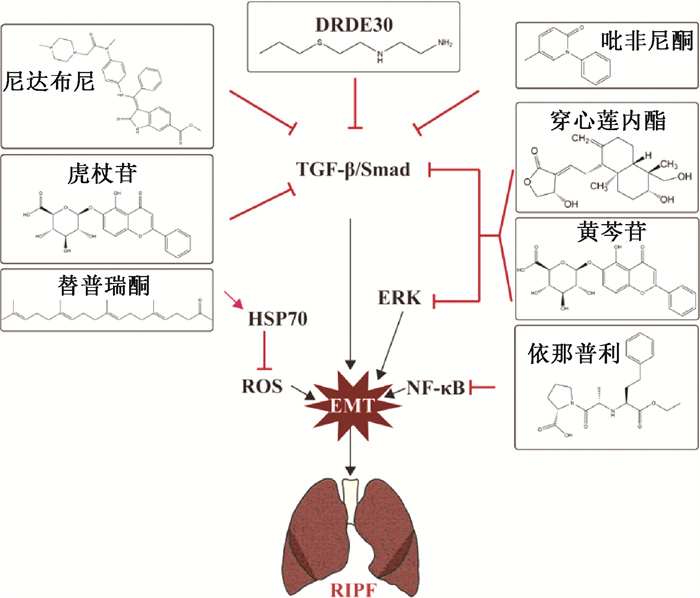
|
图 2 放射性肺纤维化中的EMT靶向药物 Figure 2 EMT targeted drugs in radiation pulmonary fibrosis |
1. TGF-β信号通路抑制剂:氨磷汀是美国食品药品监督管理局(FDA)批准的一种选择性正常组织放射防护剂,被发现可以降低放射性肺炎的发生率和严重程度,但其治疗剂量不良反应限制了其实现临床转化[53]。最近的研究发现,一种不良反应更低的氨磷汀类似物DRDE 30可消除了促炎性NF-κB和p38/MAPK信号级联的激活,抑制促炎性细胞因子IL-1β、TNF-α和IL-6的释放,并通过抑制TGF-β/Smad通路介导的EMT,减少肺组织中羟脯氨酸和胶原蛋白的含量,从而抑制RIPF[54]。抗炎药吡非尼酮和酪氨酸激酶抑制剂尼达尼布是目前被批准用于特发性肺纤维化患者的治疗药物,可通过抑制TGF-β通路激活以减少博莱霉素或辐射诱导的组织纤维化[55-56]。虎杖苷是从虎杖中提取的白藜芦醇的天然前体。Cao等[57]研究表明,虎杖苷通过抑制TGF-β/Smad信号通路缓解电离辐射诱导的EMT过程和放射性肺纤维化。
2. ERK信号通路抑制剂:穿心莲内酯是从穿心莲中提取纯化的活性成分。Gao等[58]发现穿心莲内酯可以通过抑制AIM2炎症小体作用介导巨噬细胞焦亡,缓解放射性肺炎。Li等[59]还发现,穿心莲内酯可通过抑制Smad2/3和ERK1/2信号通路,来抑制TGF-β1诱导的Ⅱ型肺泡上皮细胞中的EMT。黄芩苷是从中药黄芩的干燥根中纯化的活性成分。Lu等[60]发现黄芩苷可通过TGF-β和ERK/GSK-3β信号通路减轻辐射诱导的原代Ⅱ型肺泡上皮细胞EMT。
3. 其他信号通路抑制剂:依那普利是一种血管紧张素转换酶抑制剂,可以抑制NF-κB信号通路的激活,减轻辐射引起的肺纤维化[61-62]。据报道,热休克蛋白70(HSP70)可通过抑制活性氧(ROS)的产生来抑制EMT[63]。替普瑞酮(geranylgeranlyacetone, GGA)是一种无毒的抗溃疡药物。Kim等[14]发现其可通过诱导人肺上皮细胞中HSP70表达抑制放射引起的EMT标志物改变。
4. 基于小分子核酸的基因治疗:miRNA作为核酸小分子,可以靶向mRNA在转录后水平发挥基因沉默,具有高度特异性,因此,在靶向基因治疗中具备较大潜力。研究表明,通过腺病毒搭载miRNA鼻吸入给药可使miRNA在肺组织中稳定表达,并有效缓解小鼠放射性肺纤维化[5-6]。除此之外,Li等[64]发现间充质干细胞衍生的miRNA也可以靶向抑制电离辐射诱导肺EMT过程。
四、结论与展望电离辐射诱导肺EMT是RIPF发生发展中的关键一环,具有可逆性,通过靶向抑制或逆转EMT可有效抑制RIPF的进程。由于缺少有效标靶,目前没有被批准用于RIPF常规临床治疗的药物。因此,积极寻找临床放疗中能够防治RIPF的潜在靶点具有十分重要的意义。
EMT是一个复杂的生物过程,涉及多因子、多信号通路,其中包括经典的TGF-β通路、ERK通路、NF-κB通路、Notch1通路以及PI3K/AKT通路等。这些信号通路被部分证实能够被电离辐射激活,因此,相关通路抑制剂对EMT和RIPF的干预作用研究最为广泛[65]。除小分子抑制剂外,一些中药成分多具有抗氧化、抗肿瘤和抗炎等多种药理作用,在放射性肺损伤防治中有较大应用前景,受到越来越多研究者的关注。核酸药物能够以化学药物或抗体药物无法靶向的分子作为作用靶点,有望对传统药物疗效不佳的疾病产生突破性的进展。因此,基于小分子核酸的基因疗法在RIPF的临床治疗中具有巨大潜力,且相较于病毒载体或纳米材料的毒性和免疫原性,自体细胞分泌的外泌体或许更可能成为临床应用的核酸载体。主要应用于肿瘤免疫治疗的DNA甲基化抑制剂也被报道在治疗组织纤维化中起重要作用[66-67],但在RIPF治疗中尚缺少相关研究。此外,最新的研究发现益生菌群在防治RIPF方面也具有重要作用。Ju等[68]发现鼠李糖乳杆菌(Lactobacillus rhamnosus GG, LGG)可下调辐射响应的lncRNA SNHG17,靶向抑制PTBP1/Notch1信号通路,从而减轻EMT和小鼠RIPF。有报道肺组织细胞外基质衍生的水凝胶也可以有效阻断或逆转电离辐射诱导肺EMT过程,其具体机制尚不清楚,可能与组织负反馈调节有关[69]。这些也给放射性肺纤维化的治疗策略带来新思路。
综上,尽管电离辐射诱导EMT调控通路与肿瘤相关EMT调控通路有交叉,但由于电离辐射诱导的组织微环境与肿瘤微环境、正常细胞和肿瘤细胞对电离辐射的响应性存在差异,电离辐射诱导肺EMT的具体机制还需要更多的证据,以寻找更具有效性和特异性的RIPF治疗靶点。与此同时,研发靶向EMT预防RIPF的药物,提出新的治疗策略如小分子抑制剂、基于小分子核酸的基因治疗以及益生菌治疗等具有重要的临床应用价值。
利益冲突 无
作者贡献声明 敖兴坤负责文献调研、撰写论文;严紫艳负责设计论文框架、论文修订;顾永清指导修改论文审校
| [1] |
Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, et al. Radiation-induced lung injury: current evidence[J]. BMC Pulm Med, 2021, 21(1): 9. DOI:10.1186/s12890-020-01376-4 |
| [2] |
Chen Z, Wu Z, Ning W. Advances in molecular mechanisms and treatment of radiation-induced pulmonary fibrosis[J]. Transl Oncol, 2019, 12(1): 162-169. DOI:10.1016/j.tranon.2018.09.009 |
| [3] |
Liu Z, Liang X, Li X, et al. miRNA-21 functions in ionizing radiation-induced epithelium-to-mesenchymal transition (EMT) by downregulating PTEN[J]. Toxicol Res (Camb), 2019, 8(3): 328-340. DOI:10.1039/c9tx00019d |
| [4] |
Wang D, Liu Z, Yan Z, et al. miRNA-155-5p inhibits epithelium-to-mesenchymal transition (EMT) by targeting GSK-3β during radiation-induced pulmonary fibrosis[J]. Arch Biochem Biophys, 2021, 697: 108699. DOI:10.1016/j.abb.2020.108699 |
| [5] |
Liang X, Yan Z, Wang P, et al. Irradiation activates MZF1 to inhibit miR-541-5p expression and promote epithelial-mesenchymal transition (EMT) in radiation-induced pulmonary fibrosis (RIPF) by upregulating slug[J]. Int J Mol Sci, 2021, 22(21): 11309. DOI:10.3390/ijms222111309 |
| [6] |
Yan Z, Ao X, Liang X, et al. Transcriptional inhibition of miR-486-3p by BCL6 upregulates Snail and induces epithelial-mesenchymal transition during radiation-induced pulmonary fibrosis[J]. Respir Res, 2022, 23(1): 104. DOI:10.1186/s12931-022-02024-7 |
| [7] |
Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016[J]. Cell, 2016, 166(1): 21-45. DOI:10.1016/j.cell.2016.06.028 |
| [8] |
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition[J]. J Clin Invest, 2009, 119(6): 1420-1428. DOI:10.1172/jci39104 |
| [9] |
Stone RC, Pastar I, Ojeh N, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis[J]. Cell Tissue Res, 2016, 365(3): 495-506. DOI:10.1007/s00441-016-2464-0 |
| [10] |
Plygawko AT, Kan S, Campbell K. Epithelial-mesenchymal plasticity: emerging parallels between tissue morphogenesis and cancer metastasis[J]. Philos Trans R Soc Lond B Biol Sci, 2020, 375(1809): 20200087. DOI:10.1098/rstb.2020.0087 |
| [11] |
Li Q, Deng MS, Wang RT, et al. PD-L1 upregulation promotes drug-induced pulmonary fibrosis by inhibiting vimentin degradation[J]. Pharmacol Res, 2022, 187: 106636. DOI:10.1016/j.phrs.2022.106636 |
| [12] |
Marconi GD, Fonticoli L, Rajan TS, et al. Epithelial-mesenchymal transition (EMT): The type-2 EMT in wound healing, tissue regeneration and organ fibrosis[J]. Cells, 2021, 10(7): 1587. DOI:10.3390/cells10071587 |
| [13] |
Choi SH, Hong ZY, Nam JK, et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis[J]. Clin Cancer Res, 2015, 21(16): 3716-3726. DOI:10.1158/1078-0432.Ccr-14-3193 |
| [14] |
Kim JS, Son Y, Jung MG, et al. Geranylgeranylacetone alleviates radiation-induced lung injury by inhibiting epithelial-to-mesenchymal transition signaling[J]. Mol Med Rep, 2016, 13(6): 4666-4670. DOI:10.3892/mmr.2016.5121 |
| [15] |
Lei X, Ma N, Liang Y, et al. Glucosamine protects against radiation-induced lung injury via inhibition of epithelial-mesenchymal transition[J]. J Cell Mol Med, 2020, 24(18): 11018-11023. DOI:10.1111/jcmm.15662 |
| [16] |
Park HR, Jo SK, Jung U. Ionizing radiation promotes epithelial-to-mesenchymal transition in lung epithelial cells by TGF-β-producing M2 macrophages[J]. In Vivo, 2019, 33(6): 1773-1784. DOI:10.21873/invivo.11668 |
| [17] |
Qu H, Liu L, Liu Z, et al. Blocking TBK1 alleviated radiation-induced pulmonary fibrosis and epithelial-mesenchymal transition through Akt-Erk inactivation[J]. Exp Mol Med, 2019, 51(4): 1-17. DOI:10.1038/s12276-019-0240-4 |
| [18] |
Marks LB. The pulmonary effects of thoracic irradiation[J]. Oncology (Williston Park), 1994, 8(6): 89-106. |
| [19] |
Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung[J]. Proc Am Thorac Soc, 2006, 3(4): 377-382. DOI:10.1513/pats.200601-004TK |
| [20] |
Mladenov E, Magin S, Soni A, et al. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy[J]. Front Oncol, 2013, 3: 113. DOI:10.3389/fonc.2013.00113 |
| [21] |
Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury[J]. Cancer Lett, 2012, 327(1-2): 48-60. DOI:10.1016/j.canlet.2011.12.012 |
| [22] |
Zimmerman MA, Huang Q, Li F, et al. Cell death-stimulated cell proliferation: a tissue regeneration mechanism usurped by tumors during radiotherapy[J]. Semin Radiat Oncol, 2013, 23(4): 288-295. DOI:10.1016/j.semradonc.2013.05.003 |
| [23] |
Graves PR, Siddiqui F, Anscher MS, et al. Radiation pulmonary toxicity: from mechanisms to management[J]. Semin Radiat Oncol, 2010, 20(3): 201-207. DOI:10.1016/j.semradonc.2010.01.010 |
| [24] |
Provatopoulou X, Athanasiou E, Gounaris A. Predictive markers of radiation pneumonitis[J]. Anticancer Res, 2008, 28(4c): 2421-2432. |
| [25] |
Kelsey CR, Horwitz ME, Chino JP, et al. Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: an assessment of risk factors[J]. Int J Radiat Oncol Biol Phys, 2011, 81(3): 812-818. DOI:10.1016/j.ijrobp.2010.06.058 |
| [26] |
Green DM, Lange JM, Qu A, et al. Pulmonary disease after treatment for Wilms tumor: a report from the national wilms tumor long-term follow-up study[J]. Pediatr Blood Cancer, 2013, 60(10): 1721-1726. DOI:10.1002/pbc.24626 |
| [27] |
Desai MY, Karunakaravel K, Wu W, et al. Pulmonary fibrosis on multidetector computed tomography and mortality in patients with radiation-associated cardiac disease undergoing cardiac surgery[J]. J Thorac Cardiovasc Surg, 2014, 148(2): 475-481.e3. DOI:10.1016/j.jtcvs.2013.08.087 |
| [28] |
Nagaraja SS, Nagarajan D. Radiation-induced pulmonary epithelial-mesenchymal transition: a review on targeting molecular pathways and mediators[J]. Curr Drug Targets, 2018, 19(10): 1191-1204. DOI:10.2174/1389450119666180207092234 |
| [29] |
Sato S, Yanagihara T, Kolb MRJ. Therapeutic targets and early stage clinical trials for pulmonary fibrosis[J]. Expert Opin Investig Drugs, 2019, 28(1): 19-28. DOI:10.1080/13543784.2019.1554054 |
| [30] |
Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis[J]. Nat Rev Nephrol, 2016, 12(6): 325-338. DOI:10.1038/nrneph.2016.48 |
| [31] |
Zhao J, Liu J, Lee JF, et al. TGF-β/SMAD3 pathway stimulates sphingosine-1 phosphate receptor 3 expression: implication of sphingosine-1 phosphate receptor 3 in lung adenocarcinoma progression[J]. J Biol Chem, 2016, 291(53): 27343-27353. DOI:10.1074/jbc.M116.740084 |
| [32] |
Chang N, Ge J, Xiu L, et al. HuR mediates motility of human bone marrow-derived mesenchymal stem cells triggered by sphingosine 1-phosphate in liver fibrosis[J]. J Mol Med (Berl), 2017, 95(1): 69-82. DOI:10.1007/s00109-016-1460-x |
| [33] |
Wang P, Luo ML, Song E, et al. Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway[J]. Sci Transl Med, 2018, 10(462): eaat2039. DOI:10.1126/scitranslmed.aat2039 |
| [34] |
Gong L, Wu X, Li X, et al. S1PR3 deficiency alleviates radiation-induced pulmonary fibrosis through the regulation of epithelial-mesenchymal transition by targeting miR-495-3p[J]. J Cell Physiol, 2020, 235(3): 2310-2324. DOI:10.1002/jcp.29138 |
| [35] |
Xie L, Law BK, Chytil AM, et al. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro[J]. Neoplasia, 2004, 6(5): 603-610. DOI:10.1593/neo.04241 |
| [36] |
Nagarajan D, Melo T, Deng Z, et al. ERK/GSK3β/Snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition[J]. Free Radic Biol Med, 2012, 52(6): 983-992. DOI:10.1016/j.freeradbiomed.2011.11.024 |
| [37] |
Kondylis V, Kumari S, Vlantis K, et al. The interplay of IKK, NF-κB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation[J]. Immunol Rev, 2017, 277(1): 113-127. DOI:10.1111/imr.12550 |
| [38] |
Kang AR, Cho JH, Lee NG, et al. RIP1 is a novel component of γ-ionizing radiation-induced invasion of non-small cell lung cancer cells[J]. Int J Mol Sci, 2020, 21(13): 4584. DOI:10.3390/ijms21134584 |
| [39] |
Kang AR, Cho JH, Lee NG, et al. Radiation-induced IL-1β expression and secretion promote cancer cell migration/invasion via activation of the NF-κB-RIP1 pathway[J]. Biochem Biophys Res Commun, 2021, 534: 973-979. DOI:10.1016/j.bbrc.2020.10.057 |
| [40] |
Kim JY, Jeon S, Yoo YJ, et al. The Hsp27-mediated ikbα-NFκB signaling axis promotes radiation-induced lung fibrosis[J]. Clin Cancer Res, 2019, 25(17): 5364-5375. DOI:10.1158/1078-0432.Ccr-18-3900 |
| [41] |
Meyer SM, Williams CC, Akahori Y, et al. Small molecule recognition of disease-relevant RNA structures[J]. Chem Soc Rev, 2020, 49(19): 7167-7199. DOI:10.1039/d0cs00560f |
| [42] |
Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms[J]. Nat Rev Genet, 2009, 10(5): 295-304. DOI:10.1038/nrg2540 |
| [43] |
Gao LM, Xu SF, Zheng Y, et al. Long non-coding RNA H19 is responsible for the progression of lung adenocarcinoma by mediating methylation-dependent repression of CDH1 promoter[J]. J Cell Mol Med, 2019, 23(9): 6411-6428. DOI:10.1111/jcmm.14533 |
| [44] |
Liu J, Sun X, Qin S, et al. CDH1 promoter methylation correlates with decreased gene expression and poor prognosis in patients with breast cancer[J]. Oncol Lett, 2016, 11(4): 2635-2643. DOI:10.3892/ol.2016.4274 |
| [45] |
Wu CH, Tang SC, Wang PH, et al. Nickel-induced epithelial-mesenchymal transition by reactive oxygen species generation and E-cadherin promoter hypermethylation[J]. J Biol Chem, 2012, 287(30): 25292-25302. DOI:10.1074/jbc.M111.291195 |
| [46] |
Fukagawa A, Ishii H, Miyazawa K, et al. δEF1 associates with DNMT1 and maintains DNA methylation of the E-cadherin promoter in breast cancer cells[J]. Cancer Med, 2015, 4(1): 125-135. DOI:10.1002/cam4.347 |
| [47] |
Galván JA, Helbling M, Koelzer VH, et al. TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer[J]. Oncotarget, 2015, 6(2): 874-885. DOI:10.18632/oncotarget.2716 |
| [48] |
Dong C, Wu Y, Yao J, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer[J]. J Clin Invest, 2012, 122(4): 1469-1486. DOI:10.1172/jci57349 |
| [49] |
Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease[J]. Nat Rev Genet, 2007, 8(11): 829-833. DOI:10.1038/nrg2218 |
| [50] |
Au Yeung WK, Brind' Amour J, Hatano Y, et al. Histone H3K9 methyltransferase G9a in oocytes is essential for preimplantation development but dispensable for CG methylation protection[J]. Cell Rep, 2019, 27(1): 282-293.e4. DOI:10.1016/j.celrep.2019.03.002 |
| [51] |
Nagaraja SS, Subramanian U, Nagarajan D. Radiation-induced H3K9 methylation on E-cadherin promoter mediated by ROS/Snail axis: Role of G9a signaling during lung epithelial-mesenchymal transition[J]. Toxicol In Vitro, 2021, 70: 105037. DOI:10.1016/j.tiv.2020.105037 |
| [52] |
Ligresti G, Caporarello N, Meridew JA, et al. CBX5/G9a/H3K9me-mediated gene repression is essential to fibroblast activation during lung fibrosis[J]. JCI Insight, 2019, 5(12): e127111. DOI:10.1172/jci.insight.127111 |
| [53] |
Devine A, Marignol L. Potential of amifostine for chemoradiotherapy and radiotherapy-associated toxicity reduction in advanced NSCLC: a meta-analysis[J]. Anticancer Res, 2016, 36(1): 5-12. |
| [54] |
Arora A, Bhuria V, Singh S, et al. Amifostine analog, DRDE-30, alleviates radiation induced lung damage by attenuating inflammation and fibrosis[J]. Life Sci, 2022, 298: 120518. DOI:10.1016/j.lfs.2022.120518 |
| [55] |
Inomata M, Kamio K, Azuma A, et al. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis[J]. Respir Res, 2014, 15(1): 16. DOI:10.1186/1465-9921-15-16 |
| [56] |
Citrin DE, Prasanna PGS, Walker AJ, et al. Radiation-induced fibrosis: mechanisms and opportunities to mitigate. Report of an NCI Workshop, September 19, 2016[J]. Radiat Res, 2017, 188(1): 1-20. DOI:10.1667/rr14784.1 |
| [57] |
Cao K, Lei X, Liu H, et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial-mesenchymal transition[J]. J Cell Mol Med, 2017, 21(12): 3264-3276. DOI:10.1111/jcmm.13230 |
| [58] |
Gao J, Peng S, Shan X, et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis[J]. Cell Death Dis, 2019, 10(12): 957. DOI:10.1038/s41419-019-2195-8 |
| [59] |
Li J, Liu J, Yue W, et al. Andrographolide attenuates epithelial-mesenchymal transition induced by TGF-β1 in alveolar epithelial cells[J]. J Cell Mol Med, 2020, 24(18): 10501-10511. DOI:10.1111/jcmm.15665 |
| [60] |
Lu J, Zhong Y, Lin Z, et al. Baicalin alleviates radiation-induced epithelial-mesenchymal transition of primary type Ⅱ alveolar epithelial cells via TGF-β and ERK/GSK3β signaling pathways[J]. Biomed Pharmacother, 2017, 95: 1219-1224. DOI:10.1016/j.biopha.2017.09.037 |
| [61] |
Ortiz LA, Champion HC, Lasky JA, et al. Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-kappaB and AP-1[J]. Am J Physiol Lung Cell Mol Physiol, 2002, 282(6): L1209-L1221. DOI:10.1152/ajplung.00144.2001 |
| [62] |
Gao F, Narayanan J, Joneikis C, et al. Enalapril mitigates focal alveolar lesions, a histological marker of late pulmonary injury by radiation to the lung[J]. Radiat Res, 2013, 179(4): 465-474. DOI:10.1667/rr3127.1 |
| [63] |
Liu J, Bao J, Hao J, et al. HSP70 inhibits high glucose-induced Smad3 activation and attenuates epithelial-to-mesenchymal transition of peritoneal mesothelial cells[J]. Mol Med Rep, 2014, 10(2): 1089-1095. DOI:10.3892/mmr.2014.2279 |
| [64] |
Li Y, Shen Z, Jiang X, et al. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3β pathway via c-MET in radiation-induced lung injury[J]. J Exp Clin Cancer Res, 2022, 41(1): 128. DOI:10.1186/s13046-022-02351-z |
| [65] |
Wang P, Yan Z, Zhou PK, et al. The promising therapeutic approaches for radiation-induced pulmonary fibrosis: targeting radiation-induced mesenchymal transition of alveolar type Ⅱ epithelial cells[J]. Int J Mol Sci, 2022, 23(23): 15014. DOI:10.3390/ijms232315014 |
| [66] |
Gros C, Fahy J, Halby L, et al. DNA methylation inhibitors in cancer: recent and future approaches[J]. Biochimie, 2012, 94(11): 2280-2296. DOI:10.1016/j.biochi.2012.07.025 |
| [67] |
Myasoedova VA, Sukhorukov V, Grechko AV, et al. Inhibitors of DNA methylation and histone deacetylation as epigenetically active drugs for anticancer therapy[J]. Curr Pharm Des, 2019, 25(6): 635-641. DOI:10.2174/1381612825666190405144026 |
| [68] |
Ju Z, Pan H, Qu C, et al. Lactobacillus rhamnosus GG ameliorates radiation-induced lung fibrosis via lncRNASNHG17/PTBP1/NICD axis modulation[J]. Biol Direct, 2023, 18(1): 2. DOI:10.1186/s13062-023-00357-x |
| [69] |
Zhou J, Wu P, Sun H, et al. Lung tissue extracellular matrix-derived hydrogels protect against radiation-induced lung injury by suppressing epithelial-mesenchymal transition[J]. J Cell Physiol, 2020, 235(3): 2377-2388. DOI:10.1002/jcp.29143 |
 2023, Vol. 43
2023, Vol. 43


