非小细胞肺癌(non-small cell lung cancer,NSCLC)初诊时约60%发生转移[1]。药物治疗是晚期NSCLC治疗的基石,包括化疗、靶向、免疫治疗。晚期NSCLC的失败模式分析,不论是化疗、靶向还是免疫治疗时代,原发肿瘤都是主要的失败部位,说明积极控制原发病灶对改善晚期NSCLC患者的生存具有重要意义[2-4]。
研究表明,在化疗、靶向或免疫治疗的基础上给予原发肿瘤的放射治疗能够延长患者的生存时间。前期多中心、前瞻性研究表明,晚期NSCLC化疗联合原发肿瘤放疗的中位总生存期(overall survival,OS)和无疾病进展生存期(progression-free survival,PFS)分别为13和9个月,疗效优于单纯化疗[5]。对于表皮生长因子受体(epidermal growth factor receptor, EGFR)敏感突变的患者,EGFR-酪氨酸激酶抑制剂(TKI)治疗有效的基础上,原发肿瘤放疗能够延长OS和PFS[6]。免疫治疗时代,晚期NSCLC胸部放疗也有很大潜力。Kharouta等[7]对美国国家癌症数据库(NCDB)接受免疫治疗的晚期NSCLC的数据分析,加入胸部放疗提高了2年生存率。
晚期NSCLC异质性强,如何筛选出能从胸部原发肿瘤中获益的患者,避免过度治疗或治疗不足是临床需要考量的关键问题。Nomogram(列线图)模型对多个预测指标进行整合,是多因素预测模型的可视化途径,可用于预测肿瘤患者预后和疗效[8]。本研究分析晚期NSCLC化疗联合胸部放疗长期生存患者的临床特征并构建Nomogram模型,以期为晚期NSCLC原发肿瘤放疗的治疗决策选择提供一些参考。
资料与方法1.研究对象:训练集病例筛选自2003年1月至2012年5月晚期NSCLC化疗同期联合胸部原发灶放疗的两项前瞻性数据[5, 9];验证集病例筛选自贵州省医科大学附属肿瘤医院2014年1月至2020年8月收治的138例符合入组标准患者。入组标准:病理证实的初治NSCLC;初诊时伴远处转移;一线治疗采用化疗,整个病程中未接受分子靶向治疗或免疫检测点抑制剂治疗;年龄18~75岁;KPS评分≥70;接受至少2个周期化疗;胸部原发病灶放疗生物学等效剂量(biologically effective dose,BED)≥45 Gy。
2.临床和实验室数据收集:临床病理信息包括临床信息包括性别、年龄、肿瘤病理类型、T期、N期、转移状态、KPS评分、原发肿瘤大小、放化疗情况和生存结果等。实验室检测指标包括治疗前1周内的白细胞(WBC)计数、血红蛋白(Hb)水平、血小板(PLT)计数、中性粒细胞绝对值、中性粒细胞/(白细胞-中性粒细胞)(dNLR)、乳酸脱氢酶(LDH)、血浆D-二聚体、纤维蛋白原。
3.肺免疫预后指数(LIPI)评分:LIPI是基于治疗前患者外周血的中性粒细胞/(白细胞-中性粒细胞) (dNLR)和乳酸脱氢酶(LDH)水平的一种复合指标。LDH以正常值高限为标准,dNLR以3为标准,其中一项异常为中(评分1分),两项均异常为差(评分2分),两项指标均正常则LIPI评分为良好(评分0分)。本研究中,LIPI为良好组152例(58.5%)、中组70例(26.9%)、差组38例(14.6%),中位总生存时间(overall survival, OS)分别为16.0、11.0和12.0个月,因LIPI评分中组与差组病例较少,且两组生存时间相似,将中组和差组合并为同一组(不良组)。
4.长期生存(long-term survival,LTS)定义:根据文献报道,晚期NSCLC单纯化疗的中位生存时间为8~10个月[10-11],化疗联合原发肿瘤放疗的中位OS为13~17个月[5, 12],因此本研究将OS≥18个月定义为长期生存。
5.治疗方法:一线化疗采用顺铂+多西他赛的两药联合化疗,21~28 d为1个周期。晚期NSCLC胸部原发肿瘤放疗的时机尚有争议,本研究的病例筛选自两项前瞻性的数据,在该两项前瞻性研究中的胸部放疗在化疗1个周期内开始,完成胸部放疗后,疗效评价为疾病控制(完全缓解、部分缓解或稳定)的患者继续完成化疗至共计4~6个周期,不进行维持治疗。原发肿瘤的放射治疗采用三维适形(3D-CRT)或调强放疗(IMRT)。胸部原发肿瘤GTV包括CT上显示的胸部原发肿瘤、纵隔淋巴引流区短径>1 cm淋巴结;CTV为GTV外放6.0 mm;PTV在CTV外放5.0~10.0 mm。要求100%处方剂量线包全98%~100%GTV,95%处方剂量线包括98%~100%CTV,90%处方剂量线包括98%~100%PTV。正常全肺体积为全肺体积减去GTV,要求肺V20≤32%,脊髓Dmax≤50 Gy,食管Dmean≤35 Gy,心脏Dmean≤30 Gy。所有入组患者的胸部原发肿瘤接受放疗剂量的BED≥45 Gy。
6.预测模型的建立及验证:Nomogram用于建立晚期NSCLC胸部放疗长期生存预测模型,每个独立的获益因素对应于各自的分数,根据该分数可以获得不同患者的总分数。晚期非小细胞肺癌胸部放疗长期生存的概率可以根据总得分的相应风险值来估计,表示事件发生的概率其范围从0到1。以区分度及校准度作为模型评价指标,一致性指数(C-index, C指数)用于评估预测和辨别的性能,受试者工作特征(receiver operating characteristic, ROC)曲线显示了每个风险因素的预测能力及Nomogram的预测能力,并列出了ROC曲线下面积(area under curve,AUC)值。校准曲线反映了预测长期生存概率与实际长期生存概率之间的关系,横坐标是预测概率,纵坐标是患者的实际概率。决策曲线(decision curve analysis, DCA)用于评估Nomogram模型的临床应用价值。
7.统计学处理:采用SPSS 26.0软件进行分析,连续变量采用ROC曲线确定最佳分组界值;计数资料组间比较采用卡方检验;logistic回归模型行多因素分析,P < 0.05为差异有统计学意义,纳入构建Nomogram模型。R语言3.6.3版用于建立Nomogram、校准曲线、ROC曲线和DCA曲线分析。
结果1.一般临床资料:训练集纳入的260例患者中位OS时间为13.4个月(95% CI:11.9~14.9),1、2和3年的OS率分别为55.4%、19.1%和11.9%。其中,LTS组87例、非LTS组173例,男性164例、女性96例,中位年龄58岁(26~75岁),196例(75.4%)的转移器官数≤2个;骨转移是最常见的转移部位(49.2%)、其次为脑(36.5%)和肺转移(31.5%)。
2.影响LTS的因素分析:单因素分析显示,KPS评分、T分期、转移器官数、转移病灶数、脑转移、骨转移、血红蛋白水平、血小板计数、纤维蛋白原、D-二聚体、乳酸脱氢酶、LIPI、BED及化疗周期数与LTS相关(χ2=4.72~12.63,P < 0.05,表 1),与性别、年龄、N分期、病理类型、肝转移、肺转移、肿瘤最大径、放疗技术、转移灶是否放疗、白细胞计数、白蛋白、中性粒细胞数、dNLR无关(P>0.05)。Logistic回归分析显示,BED、化疗周期数、PLT计数、D-二聚体及LIPI评分是影响LTS的独立预测因素(表 2)。
|
|
表 1 影响LTS的单因素分析 Table 1 Univariate analysis of factors affecting LTS |
|
|
表 2 影响LTS的logstic回归多因素分析 Table 2 Multivariate logistic regression analysis of factors affecting LTS |
2. Nomogram预测模型的构建及验证:基于多因素分析中LTS的独立预测因素(化疗周期数、BED、PLT计数、D-二聚体及LIPI评分)构建Nomogram模型(图 1)。收集2014年1月至2020年8月符合入组标准的138例患者的临床资料对Nomogram模型进行验证,验证集患者的中位OS时间为12.0个月(95% CI 9.9~14.1),1、2和3年的OS率分别为52.4%、21.2%和11.3%。其中,LTS组64例,非LTS组74例;男性106例,女性32例,中位年龄59岁(33~78岁)。结果显示,LTS与KPS、转移器官数、肿瘤最大径、化疗周期数、原发肿瘤放疗剂量、白细胞计数、血小板计数、纤维蛋白原、LIPI有关(χ2=4.09~18.74,P < 0.05),与性别、年龄、T分期、N分期、病例类型、转移病灶数、脑转移、骨转移、肝转移、肺转移、放疗技术、转移灶放疗、血红蛋白水平、白蛋白水平、中性粒细胞计数、D-二聚体、乳酸脱氢酶、dNLR无关(P>0.05),验证集患者的临床特征详见表 3。
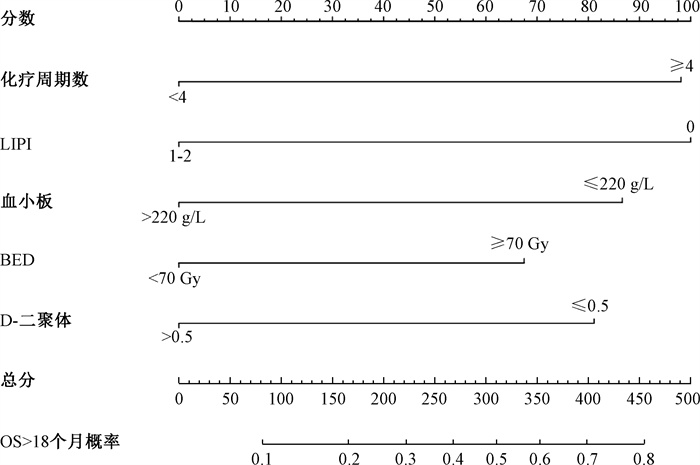
|
注:LIPI.肺免疫预后指数;BED.生物有效剂量;OS.总生存 图 1 晚期NSCLC胸部放疗长期生存Nomogram模型 Figure 1 Nomogram model of the long-term survival patients with advanced NSCLC treated with thoracic radiotherapy |
|
|
表 3 验证集患者的临床资料特征 Table 3 Clinical characteristics of patients in the validation set |
训练队列的C指数为0.750(95%CI 0.688~0.813),验证队列的C指数为0.727(95%CI 0.641~0.813)。ROC曲线比较Nomogram和单一指标预测能力,训练队列和验证队列都表明,Nomogram具有最高的AUC值(图 2A,2B)。训练队列和验证队列的校准曲线显示预测值和实际值之间的拟合度较高(P=0.454,P=0.405),表明Nomogram能较好地预测晚期NSCLC胸部放疗长期生存概率(图 2C,2D)。训练队列(图 2E)和验证队列(图 2F)的DCA曲线也表明,Nomogram比任何单一预测因子具有更好的预测能力。
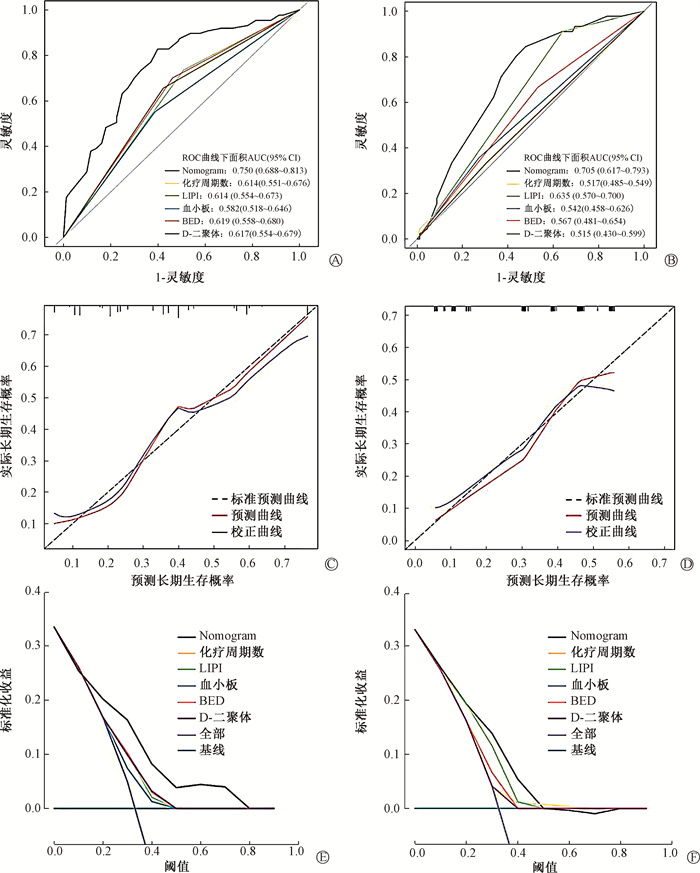
|
图 2 训练集和验证集的ROC曲线、校准曲线以及DCA曲线 A. 训练集ROC曲线;B. 验证集ROC曲线;C. 训练集校准曲线;D. 验证集校准曲线;E. 训练集DCA曲线;F. 验证集DCA曲线 Figure 2 The ROC, corrected, and DCA curves of the training and validation sets A. ROC curves of the training set; B. ROC curves of the validation set; C. Corrected curves of the training set; D. Corrected curves of the validation set; E. DCA curves of the training set; F. DCA curves of the validation set |
转移器官数和转移病灶数是影响晚期NSCLC的主要因素。1、2和3个器官转移的中位OS分别为16.0、12.4和11.0个月(χ2= 11.37,P =0.003),但组间比较发现2个器官转移与3个器官转移的OS相似(χ2= 2.04,P =0.154);转移病灶数≤5个和>5个的中位OS分别为15.3和11.3(χ2 = 12.04,P =0.001)。转移器官数和转移病灶数预测LTS的C指数分别为0.605和0.573。
讨论晚期NSCLC离不开药物治疗,从失败模式看,原发肿瘤是主要的失败部位[2-4]。晚期NSCLC原发肿瘤的放疗主要是以姑息减症为目的。近年来,越来越多的研究表明,晚期NSCLC在药物治疗的基础上给予原发肿瘤放射治疗能够延长生存期[13-16]。不可否认,药物治疗是晚期NSCLC的基石,哪些患者能从原发肿瘤的放疗中获益,值得探讨。本研究对晚期NSCLC胸部放疗长期生存患者的临床特征进行分析,结果显示化疗周期数、BED、PLT计数、D-二聚体及LIPI评分是影响LTS的独立预测因素。
药物治疗是晚期NSCLC治疗的基石,推荐的化疗4~6个周期。本研究也发现,≥4周期化疗患者的预后更好。近年来,越来越多的研究表明在药物治疗的基础上,原发肿瘤的放射治疗能给晚期NSCLC带来生存获益[6, 16]。晚期NSCLC化疗的中位OS为8.0~10个月,1年、2年生存率分别为30.0%、10.0%[17-18]。本研究中,全组患者的中位OS时间为13.4个月(95%CI 11.9~14.9),1、2和3年的OS率分别为55.4%、20.8%和14.6%。表明在化疗的基础上,给予原发肿瘤放疗能够延长OS。然而,目前尚缺少临床随机对照研究评价晚期NSCLC原发肿瘤应给予根治性放疗或姑息性放疗。本研究提示,原发肿瘤积极的放射治疗BED≥70 Gy能够带来生存获益。既往也有研究表明,更高剂量的放疗可给晚期NSCLC带来生存获益[15-16, 19]。
PLT能与肿瘤细胞聚集在一起,逃避免疫系统的识别,并附着于血管内皮细胞,促进肿瘤的转移、侵袭和生长[20]。本研究也显示,PLT升高是晚期NSCLC患者OS的不利因素。研究表明,恶性肿瘤与机体的凝血系统相互影响,机制复杂[21]。研究表明,D-二聚体升高是影响生存的不利预后因素[22]。本研究也证实,D-二聚体是影响LTS的独立预后因素。
炎性反应是肿瘤微环境的特点之一,会导致免疫功能低下和肿瘤细胞免疫逃避[23]。因此,炎症标记物可为肿瘤患者提供有价值的预后信息。LDH在人体广泛分布,存在于细胞中,是糖无氧酵解及糖异生的关键酶,当细胞受损时,释放到血液中。LDH与肿瘤的免疫炎症微环境及肿瘤负荷相关[24]。大量临床研究证实,dNLR及LDH水平与晚期NSCLC的预后相关[25-27]。近年的研究表明,基于dNLR及LDH衍生的LIPI可能是一个较好的疗效预测指标。Kazandjian等[28]发现,晚期NSCLC无论接受免疫检查点抑制剂治疗、靶向治疗或细胞毒化疗,LIPI都与OS、PFS相关,LIPI良好组的预后优于不良组。Zhang等[29]报道,对于接受放化疗的不可切除局部晚期NSCLC,LIPI是一个简便而有前景的预后标志物,LIPI良好组的生存期更长。在本研究中,多因素分析也发现LIPI与患者的长期生存相关,是影响OS的独立预后因素,但dNLR和LDH都不是影响OS的独立预后因素。这提示,在不同的治疗模式需要重新判定相关指标的预后价值,在化疗联合胸部放疗的晚期NSCLC中,LIPI是比dNLR和LDH更好的疗效预测指标。
Nomogram模型是一种简便、精确的预测的方法,能够根据患者和疾病特征来估计个体化风险,广泛运用于癌症的预后评估[30-31]。除Nomogram模型外,常用的临床预测模型还包括评分系统及热图,但这两种预测模型的连续变量必须转换为分类变量,因此预测模型的准确度会有损失[32]。Hoang等[33]最早报道了晚期NSCLC的Nomogram预后模型,但该模型基于一线化疗的晚期NSCLC患者建立。本研究构建的Nomogram模型是基于化疗联合胸部放疗的晚期NSCLC患者资料,旨在筛选出在化疗基础上能够从原发肿瘤放疗中获益的患者。Nomogram模型的预测性能可通过ROC曲线来评估,并可采用DCA曲线评估临床应用价值。本研究基于logistic回归分析结果构建的Nomogram模型,能够很好地预测晚期NSCLC胸部放疗的长期生存。通过DCA曲线及ROC曲线证实Nomogram的预测能力优于任何单一指标,临床预测的效益性更好。本研究中,Nomogram模型预测LTS的C指数高于转移器官数和转移病灶数的C指数,分别为0.750、0.605和0.573,表明Nomogram模型预测LTS的准确性更优。本研究构建Nomogram模型中的PLT、LIPI评分及D-二聚体来自血常规、血生化和凝血功能等临床常规检测项目,不给患者带来额外的痛苦和经济负担,具有较好的可操作性和适用性。
本研究亦存在以下不足:① 本研究为回顾性研究,不可避免存在选择偏倚。② 靶向治疗或免疫治疗目前是晚期NSCLC药物治疗的主要策略。本研究中,所有入组患者未接受过分子靶向或免疫治疗,需进一步评价分子靶向或免疫治疗基础上原发肿瘤放疗患者长期生存的特征。但对没有敏感基因突变的晚期NSCLC或有免疫治疗禁忌证的患者具有一定的指导价值。③ 构建的Nomogram仍受到数据收集的回顾性限制,还需在前瞻性数据收集和患者随访、更广泛的地域招募以及纳入其他因素方面进一步验证及分析,以改进该模型。
综上所述,化疗周期数、BED、PLT计数、D-二聚体及LIPI评分是影响晚期NSCLC长期生存的独立预后因素,基于这些预后因素构建的Nomogram模型为筛选胸部放疗受益患者提供了便捷、直观且个性化的预测模型。
利益冲突 所有作者均声明不存在利益冲突
作者贡献声明 姜炜负责数据收集、整理与分析、论文撰写;马筑、李青松、耿一超、罗大先、杨文刚、陈霞霞、欧阳伟炜负责实施及指导研究;苏胜发、卢冰、胡银祥负责整体设计、指导论文撰写及修改
| [1] |
Siegel R, Miller K, Fuchs H, et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. DOI:10.3322/caac.21654 |
| [2] |
Mehta N, Mauer AM, Hellman S, et al. Analysis of further disease progression in metastatic non-small cell lung cancer: implications for locoregional treatment[J]. Int J Oncol, 2004, 25(6): 1677-1683. DOI:10.3892/ijo.25.6.1677 |
| [3] |
Patel SH, Rimner A, Foster A, et al. Patterns of initial and intracranial failure in metastatic EGFR-mutant non-small cell lung cancer treated with erlotinib[J]. Lung Cancer, 2017, 108: 109-114. DOI:10.1016/j.lungcan.2017.03.010 |
| [4] |
Attia CG, Fei N, Almubarak M, et al. Patterns of disease progression to checkpoint inhibitor immunotherapy in patients with stage Ⅳ non-small cell lung cancer[J]. J Med Imaging Radiat Oncol, 2020, 64(6): 866-872. DOI:10.1111/1754-9485.13096 |
| [5] |
Su S, Li T, Lu B, et al. Three-dimensional radiation therapy to the primary tumor with concurrent chemotherapy in patients with stage Ⅳ non-small cell lung cancer: results of a multicenter phase 2 study from PPRA-RTOG, China[J]. Int J Radiat Oncol Biol Phys, 2015, 93(4): 769-777. DOI:10.1016/j.ijrobp.2015.08.012 |
| [6] |
Yen Y, Hsu H, Chang J, et al. Efficacy of thoracic radiotherapy in patients with stage ⅢB-Ⅳ epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment[J]. Radiother Oncol, 2018, 129(1): 52-60. DOI:10.1016/j.radonc.2018.03.007 |
| [7] |
Kharouta M, Gross AJ, Kelley K, et al. Analysis of patterns of care and benefit of thoracic radiotherapy for patients with stage Ⅳ NSCLC in the immunotherapy-era from a national hospital-based registry[J]. Int J Radiat Oncol Biol Phys, 2021, 111(3): 441-442. DOI:10.1200/JCO.2021.39.15_suppl.9123 |
| [8] |
Balachandran V, Gonen M, Smith J, et al. Nomograms in oncology: more than meets the eye[J]. Lancet Oncol, 2015, 16(4): 173-180. DOI:10.1016/s1470-2045(14)71116-7 |
| [9] |
Su S, Hu Y, Ouyang W, et al. Overall survival and toxicities regarding thoracic three-dimensional radiotherapy with concurrent chemotherapy for stage Ⅳ non-small cell lung cancer: results of a prospective single-center study[J]. BMC Cancer, 2013, 13: 474. DOI:10.1186/1471-2407-13-474 |
| [10] |
Scagliotti G, De Marinis F, Rinaldi M, et al. Phase Ⅲ randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer[J]. J Clinical Oncol, 2002, 20(21): 4285-4291. DOI:10.1200/jco.2002.02.068 |
| [11] |
Schiller J, Harrington D, Belani C, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer[J]. New Engl J Med, 2002, 346(2): 92-98. DOI:10.1056/NEJMoa011954 |
| [12] |
Liu L, Li Q, Hu Y, et al. Prognostic model to predict overall survival for metastatic non-small cell lung cancer patients treated with chemotherapy combined with concurrent radiation therapy to the primary tumor: analysis from two prospective studies[J]. Front Oncol, 2021, 11: 625688. DOI:10.3389/fonc.2021.625688 |
| [13] |
Wei H, Zhou X, Yang H, et al. Stereotactic body radiotherapy to the primary lung lesion improves the survival of the selected patients with non-oligometastatic NSCLC harboring EGFR activating mutation with first-line EGFR-TKIs: a real-world study[J]. J Cancer Res Clin Oncol, 2022, 148(10): 2589-2598. DOI:10.1007/s00432-021-03831-z |
| [14] |
Zheng L, Wang Y, Xu Z, et al. Concurrent EGFR-TKI and thoracic radiotherapy as First-Line treatment for stage Ⅳ non-small cell lung cancer harboring EGFR active mutations[J]. Oncologist, 2019, 24(8): 1031. DOI:10.1634/theoncologist.2019-0285 |
| [15] |
Farooqi A, Ludmir E, Mitchell K, et al. Increased biologically effective dose (BED) to the primary tumor is associated with improved survival in patients with oligometastatic NSCLC[J]. Radiother Oncol, 2021, 163: 114-118. DOI:10.1016/j.radonc.2021.08.005 |
| [16] |
Uhlig J, Case M, Blasberg J, et al. Comparison of survival rates after a combination of local treatment and systemic therapy vs systemic therapy alone for treatment of stage Ⅳ non-small cell lung cancer[J]. JAMA Netw Open, 2019, 2(8): 199702. DOI:10.1001/jamanetworkopen.2019.9702 |
| [17] |
Aarts MJ, van den Borne BE, Biesma B, et al. Improvement in population-based survival of stage Ⅳ NSCLC due to increased use of chemotherapy[J]. Int J Cancer, 2015, 136(5): 387-395. DOI:10.1002/ijc.29216 |
| [18] |
Guerra JLL, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastatic at diagnosis[J]. Int J Radiat Oncol Biol Phys, 2012, 84(1): 61-67. DOI:10.1016/j.ijrobp.2012.02.054 |
| [19] |
Lopez Guerra J, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis[J]. Int J Radiat Oncol Biol Phys, 2012, 84(1): 61-67. DOI:10.1016/j.ijrobp.2012.02.054 |
| [20] |
Zhang SS, Zhang M, Yuan L, et al. Analysis of platelet parameters and activation markers in hematologic metastases of lung cancer[J]. Chin Med J (Engl), 2019, 132(6): 735-737. DOI:10.1097/cm9.0000000000000138 |
| [21] |
Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis[J]. Blood, 2017, 130(13): 1499-1506. DOI:10.1182/blood-2017-03-743211 |
| [22] |
Zhu L, Li J, Chen P, et al. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients[J]. Clin Transl Oncol, 2016, 18(2): 178-188. DOI:10.1007/s12094-015-1350-7 |
| [23] |
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences[J]. Immunity, 2019, 51(1): 27-41. DOI:10.1016/j.immuni.2019.06.025 |
| [24] |
Ding J, Karp J, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments[J]. Cancer Biomark, 2017, 19(4): 353-363. DOI:10.3233/cbm-160336 |
| [25] |
Li B, Li C, Guo M, et al. Predictive value of LDH kinetics in bevacizumab treatment and survival of patients with advanced NSCLC[J]. Onco Targets Ther, 2018, 11: 6287-6294. DOI:10.2147/ott.S171566 |
| [26] |
Mezquita L, Preeshagul I, Auclin E, et al. Predicting immunotherapy outcomes under therapy in patients with advanced NSCLC using dNLR and its early dynamics[J]. Eur J Cancer, 2021, 151: 211-220. DOI:10.1016/j.ejca.2021.03.011 |
| [27] |
de Jong C, Deneer V, Kelder J, et al. Association between serum biomarkers CEA and LDH and response in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy[J]. Thoracic Cancer, 2020, 11(7): 1790-1800. DOI:10.1111/1759-7714.13449 |
| [28] |
Kazandjian D, Gong Y, Keegan P, et al. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer[J]. JAMA Oncol, 2019, 5(10): 1481-1485. DOI:10.1001/jamaoncol.2019.1747 |
| [29] |
Zhang T, Xue W, Wang D, et al. A validation study on the lung immune prognostic index for prognostic value in patients with locally advanced non-small cell lung cancer[J]. Radiother Oncol, 2021, 156: 244-250. DOI:10.1016/j.radonc.2020.12.039 |
| [30] |
Wang R, Dai W, Gong J, et al. Development of a novel combined nomogram model integrating deep learning-pathomics, radiomics and immunoscore to predict postoperative outcome of colorectal cancer lung metastasis patients[J]. J Hematol Oncol, 2022, 15(1): 11. DOI:10.1186/s13045-022-01225-3 |
| [31] |
Sternberg C. Are nomograms better than currently available stage groupings for bladder cancer?[J]. J Clin Oncol, 2006, 24(24): 3819-3820. DOI:10.1200/jco.2006.07.1290 |
| [32] |
Wang S, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer[J]. J Clin Oncol, 2011, 29(35): 4627-4632. DOI:10.1200/jco.2010.33.8020 |
| [33] |
Hoang T, Xu R, Schiller J, et al. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data[J]. J Clin Oncol, 2005, 23(1): 175-183. DOI:10.1200/jco.2005.04.177 |
 2023, Vol. 43
2023, Vol. 43


