鳞状细胞癌是我国食管癌患者最常见的病理类型,占90%以上[1]。尽管手术、放疗、化疗、免疫治疗等综合治疗水平不断提高,其长期生存仍较差。因此,筛选有预后作用的指标对指导治疗具有重要价值。
近年来,许多研究表明,系统炎症反应、免疫状态与多种恶性肿瘤的长期生存密切相关[2-3],中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)和单核细胞与淋巴细胞比值(MLR)等指标是食管癌、肺癌、胃癌患者预后的独立影响因素[4-7]。系统免疫炎症指数(systemic immune-inflammation index, SII)是近年来提出的一个新指标,在多种癌症中具有预测预后意义[8-12],但在接受根治性放疗的食管癌患者中的研究较少。本研究拟分析SII对食管癌根治性放疗患者预后的影响,为临床提供参考价值。
资料与方法1.临床资料:回顾性分析2011年至2017年于河北医科大学第四医院接受根治性放疗的食管癌患者的临床资料。纳入标准:经组织学或细胞学证实为食管鳞状细胞癌,需行根治性放疗,且疗前未接受化疗等抗肿瘤治疗;KPS评分≥70;既往无恶性肿瘤病史;无严重基础疾病。入组患者临床资料见表 1。
|
|
表 1 食管癌患者倾向得分匹配前后SII与临床特征的关系 Table 1 Correlation between SII and clinical characteristics of ESCC patients before and after PSM |
2. 放射治疗:入组患者接受三维适形放射治疗或调强放射治疗。根据定位CT图像,结合食管钡餐造影、内镜检查或/和PET/CT等影像学资料勾画大体肿瘤靶区(GTV),临床靶区(CTV)包括GTV上下方向外扩2 cm、轴向外扩0.5~1.0 cm,计划靶区(PTV)包括CTV外扩0.5~0.8 cm。转移淋巴结(GTVnd)勾画标准为为淋巴结短径≥1 cm,食管旁、气管食管旁沟、心膈角、腹腔淋巴结的勾画标准为长径≥0.5 cm。转移淋巴结计划靶区(PTVnd)为GTVnd外扩1.0 cm。正常组织耐受剂量限制为双肺V5≤60%、V20≤30%、V30≤20%,心脏平均剂量 < 30 Gy、V25≤50%、V40≤30%,脊髓最大剂量 < 45 Gy。处方剂量54~68 Gy,单次剂量1.8~2.0 Gy,1次/d,每周5次。
3. 化疗:根据患者一般情况、肿瘤因素及个人意愿决定化疗。化疗采用放疗同步化疗或放疗后序贯化疗。方案为LFP方案(亚叶酸钙200 mg/次,第1~5天+替加氟1.0 g/次,第1~5天+顺铂20 mg/次,第1~5天)或TP方案(紫杉醇135 mg/m2第1天+顺铂75 mg/m2第1天)。全组接受化疗者共160例,化疗疗程1~6个周期,中位4个周期。
4.计算SII:放疗前、放疗期间每周及放疗结束时均采集血常规,SII血小板计数×中性粒细胞计数/淋巴细胞计数。采用放疗前SII中位值将患者分为低SII组(SII≤648)和高SII组(SII>648)进行分析。
5.随访:随访以门诊复查为主。放疗后前2年每3个月复查1次,2年后每6个月复查1次。每次复查主要包括颈胸腹部CT、食管钡餐造影、血常规、肿瘤标记物等项目,必要时复查电子胃镜。
6. 统计学处理:采用SPSS 26.0统计软件进行数据处理,Pearson相关性分析SII与临床特征之间的关系,Kaplan-Meier法计算总生存率和无进展生存率,Cox回归模型进行预后分析,对可能影响预后的变量采用1∶1倾向得分匹配法配对后再次计算生存及预后分析。P < 0.05为差异有统计学意义。
结果1. 临床特征:本研究共纳入303例患者,其中男性178例(58.7%),女性125例(41.3%),年龄41~90岁,中位年龄67岁。放疗前SII平均值757,中位值648,范围83~4371,根据放疗前SII中位值将全组患者分为高SII组(151例)和低SII组(152例)。两组患者临床特征详见表 1。Pearson相关性分析显示SII与T分期和TNM分期显著相关(χ2=8.015、8.619,P=0.018、0.013)。
2. 生存情况:全组患者1、3、5年总生存率(OS)分别为74.9%、35.5%、25.0%,无进展生存率(PFS)分别为57.1%、27.6%、19.9%。放疗前高SII组和低SII组1、3、5年OS分别为64.9%、27.1%、19.4%和84.9%、43.9%、30.5%,PFS分别为46.4%、20.3%、13.3% 和67.8%、34.8%、26.5%。Kaplan-Meier分析显示,高SII组OS和PFS均低于低SII组(χ2=13.443、12.383,P < 0.001,图 1)。
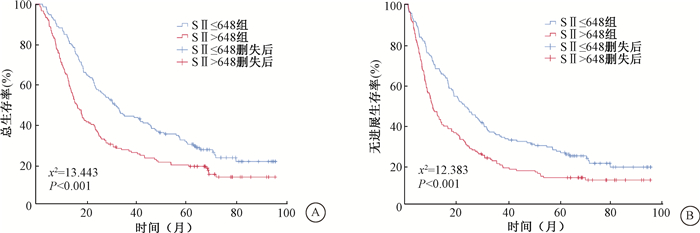
|
注:SII. 系统免疫炎症指数 图 1 303例食管癌患者不同SII分组总生存(A)和无进展生存(B)曲线 Figure 1 Overall survival (A) and progression-free survival (B) curves based on SII of 303 ESCC patients treated with radiotherapy |
3. 生存预后的单因素和多因素分析:单因素预后分析显示,年龄、肿瘤部位、T分期、淋巴结转移、放疗剂量、化疗及SII为总生存和无进展生存的影响因素。多因素预后分析显示,T分期、淋巴结转移、化疗和SII为总生存和无进展生存的独立影响因素。
4. 倾向得分匹配后SII与生存的关系:将影响两组患者生存的因素按卡钳值0.02进行1 ∶1倾向得分匹配,匹配后共有238例患者,两组间临床特征分布均衡(表 1)。Kaplan-Meier分析显示,匹配后高SII组总生存率和无进展生存率均低于低SII组差异有统计学意义(χ2=4.264、5.376,P=0.039、0.020),见图 2。
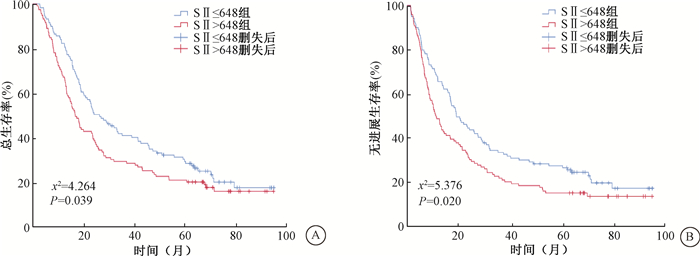
|
注:SII. 系统免疫炎症指数 图 2 倾向得分匹配后238例食管癌患者不同SII分组总生存(A)和无进展生存(B)曲线 Figure 2 Overall survival (A) and progression-free survival (B) curves based on SII of 238 ESCC patients treated with radiotherapy after PSM |
|
|
表 2 倾向得分匹配后238例食管癌患者总生存和无进展生存预后分析 Table 2 Univariate and multivariate analyses of prognostic factors for OS and PFS in 238 ESCC patients after PSM |
5. 倾向得分匹配后生存预后的单因素和多因素分析:倾向得分匹配后,单因素预后分析显示,年龄、T分期、淋巴结转移、放疗剂量、化疗及SII为总生存和无进展生存的影响因素。多因素预后分析显示,T分期、淋巴结转移、化疗和SII为总生存和无进展生存的独立影响因素(表4)。
讨论近年研究显示,系统性炎症与多种恶性肿瘤的预后有相关性[13-14]。研究证实,系统免疫炎症指数与肺癌、乳腺癌等术后生存密切相关[15-16],在食管癌中也证实术前SII是生存和生活质量的独立预测因子。Geng等[17]最先报道了SII在食管癌中的预后价值,通过回顾性分析916例接受手术的食管鳞癌患者,证实SII是总生存的独立危险因素,且SII的预测价值优于PLR、NLR和MLR。Wang等[18]报道,高SII与T分期、N分期和TNM分期相关,术前SII是预测食管鳞癌患者生存和生活质量的有效指标。Gao等[19]回顾性分析了468例行R0根治术的食管鳞癌患者,结果显示,SII是可手术食管鳞癌患者简单、有前景的预后预测指标,并通过比较曲线下面积,同样发现SII对预后的预测能力优于NLR、PLR和MLR。然而,对于接受根治性放疗的食管癌患者,SII预后价值的相关研究较少。本研究回顾性分析了303例接受根治性放疗的食管鳞癌患者,结果显示,SII与T分期和TNM分期显著相关,高SII组总生存率和无进展生存率均低于低SII组,SII是食管癌患者OS和PFS的独立预后影响因素。
既往关于SII与恶性肿瘤患者预后关系的研究主要是针对手术、放疗或化疗患者,而对接受免疫治疗的恶性肿瘤患者的研究较少。Liu等[10]回顾性分析了44例二线或多线应用单药纳武利尤单抗治疗的晚期/转移性非小细胞肺癌患者,结果显示,低SII患者的PFS和OS优于高SII患者,SII是接受免疫治疗的晚期非小细胞肺癌患者的独立预后预测因子。日本一项回顾性多中心队列研究分析了8家研究机构的接受纳武利尤单抗和伊匹单抗治疗的转移性肾癌患者,结果显示,高SII组患者1年PFS低于低SII组,认为SII可能有助于预测肾癌患者的临床结局[21]。目前SII与接受免疫治疗的食管癌患者预后相关性研究较少,这也提供了后续研究的思路和方向。
SII对肿瘤患者生存的影响机制尚不明确,可能与血小板、中性粒细胞和淋巴细胞的数量和功能有关。血小板计数升高可以刺激肿瘤血管生成,保护肿瘤细胞免于细胞溶解,从而促进肿瘤的进展和转移[21-22]。中性粒细胞通过释放大量细胞因子,改变肿瘤微环境,促进肿瘤细胞增殖和转移,还可能导致T细胞活化紊乱,帮助癌细胞逃避免疫监视[23]。淋巴细胞通过分泌因子和诱导细胞毒性细胞死亡,参与肿瘤细胞的破坏和凋亡,是机体抗肿瘤免疫反应的主要成员[24]。因此,SII同时反映了机体的炎症状态和免疫功能,高SII意味着肿瘤患者全身炎症反应较强,而免疫防御较弱,有可能影响肿瘤患者的生存,在临床实践中有望成为有效的生物标志物来预测肿瘤患者的预后,为个体化治疗策略的选择提供帮助。
综上所述,SII是接受根治性放疗食管癌患者的独立预后因素。基于简单、经济等特点,SII可作为食管癌患者预后的有效预测指标。本研究为单中心回顾性研究,尽管采用了倾向得分匹配,但仍难免选择偏倚,今后仍需前瞻性大样本研究进一步验证。
利益冲突 所有作者未因进行该研究而接受任何不正当的职务或财务利益,在此对研究的独立性和科学性予以保证
作者贡献声明 赵彦负责整理数据、统计分析及论文撰写;沈文斌协助修改论文;李娟、宋春洋负责统计分析;王旋负责收集整理病历资料;祝淑钗负责研究设计和论文修改
| [1] |
Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China[J]. J Epidemiol, 2013, 23(4): 233-242. DOI:10.2188/jea.je20120162 |
| [2] |
Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal[J]. Immunol Rev, 2016, 273: 329-343. DOI:10.1111/imr.12459 |
| [3] |
Liu X, Chen S, Liu J, et al. Impact of systemic inflammation on gastric cancer outcomes[J]. PLoS One, 2017, 12(3): e0174085. DOI:10.1371/journal.pone.0174085 |
| [4] |
Duan H, Zhang X, Wang FX, et al. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma[J]. World J Gastroenterol, 2015, 21(18): 5591-5597. DOI:10.1245/s10434-015-4869-5 |
| [5] |
Zhi X, Jiang K, Shen Y, et al. Peripheral blood cell count ratios are predictive biomarkers of clinical response and prognosis for non-surgical esophageal squamous cell carcinoma patients treated with radiotherapy[J]. J Clin Lab Anal, 2020, 34(10): e23468. DOI:10.1002/jcla.23468 |
| [6] |
Mandaliya H, Jones M, Oldmeadow C, et al. Prognostic biomarkers in stage Ⅳ non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI)[J]. Transl Lung Cancer Res, 2019, 8(6): 886-894. DOI:10.21037/tlcr.2019.11.16 |
| [7] |
Zhao R, Shan J, Nie L, et al. The predictive value of the ratio of the product of neutrophils and hemoglobin to lymphocytes in non-muscular invasive bladder cancer patients with postoperative recurrence[J]. J Clin Lab Anal, 2021, 35(8): e23883. DOI:10.1002/jcla.23883 |
| [8] |
Wang BL, Tian L, Gao XH, et al. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection[J]. Clin Chem Lab Med, 2016, 54(12): 1963-1969. DOI:10.1515/cclm-2015-1191 |
| [9] |
Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer[J]. World J Gastroenterol, 2017, 23: 6261. DOI:10.3748/wjg.v23.i34.6261 |
| [10] |
Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab[J]. J Clin Lab Anal, 2019, 33(8): 22964. DOI:10.1002/jcla.22964 |
| [11] |
Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SⅡ) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus[J]. Medicine (Baltimore), 2017, 96(4): e5886. DOI:10.1097/MD.0000000000005886 |
| [12] |
Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune‐inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma[J]. J Cell Physiol, 2019, 234: 1794-1802. DOI:10.1002/jcp.27052 |
| [13] |
Sherry AD, von Eyben R, Newman NB, et al. Systemic inflammation after radiation predicts locoregional recurrence, progression, and mortality in stage Ⅱ-Ⅲ triple-negative breast cancer[J]. Int J Radiat Oncol Biol Phys, 2020, 108(1): 268-276. DOI:10.1016/j.ijrobp.2019.11.398 |
| [14] |
Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance[J]. World J Gastroenterol, 2019, 25(31): 4383-4404. DOI:10.3748/wjg.v25.i31.4383 |
| [15] |
Shoji F. Clinical impact of the systemic immune-inflammation index in non-small cell lung cancer patients[J]. Ann Transl Med, 2020, 8(11): 668. DOI:10.21037/atm.2020.03.180 |
| [16] |
Hua X, Long ZQ, Zhang YL, et al. Prognostic value of preoperative systemic immune-inflammation index in breast cancer: a propensity score-matching study[J]. Front Oncol, 2020, 10: 580. DOI:10.3389/fonc.2020.00580 |
| [17] |
Geng Y, Shao Y, Zhu D, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis[J]. Sci Rep, 2016, 6: 39482. DOI:10.1038/srep39482 |
| [18] |
Wang L, Wang C, Wang J, et al. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma[J]. J Cancer Res Clin Oncol, 2017, 143: 2077-2086. DOI:10.1007/s00432-017-2451-1 |
| [19] |
Gao Y, Guo W, Cai S, et al. Systemic immune-inflammation index (SⅡ) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma[J]. J Cancer, 2019, 10(14): 3188-3196. DOI:10.7150/jca.30281 |
| [20] |
Iinuma K, Enomoto T, Kwada K, et al. Utility of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index as prognostic, predictive biomarkers in patients with metastatic renal cell carcinoma treated with nivolumab and ipilimumab[J]. J Clin Med, 2021, 10(22): 5325. DOI:10.3390/jcm10225325 |
| [21] |
Jain S, Harris J, Ware J, et al. Platelets: linking hemostasis and cancer[J]. Arterioscler Thromb Vasc Biol, 2010, 30(12): 2362-2367. DOI:10.1161/ATVBAHA.110.207514 |
| [22] |
Wojtukiewicz MZ, Sierko E, Hempel D, et al. V. Platelets and cancer angiogenesis nexus[J]. Cancer Metastasis Rev, 2017, 36(2): 249-262. DOI:10.1007/s10555-017-9673-1 |
| [23] |
Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity[J]. Nat Rev Immunol, 2011, 11(8): 519-531. DOI:10.1038/nri3024 |
| [24] |
Fang H, Declerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials[J]. Cancer Res, 2013, 73: 4965-4977. DOI:10.1158/0008-5472.CAN-13-0661 |
 2022, Vol. 42
2022, Vol. 42


