神经内分泌肿瘤(neuroendocrine tumors,NETs)[1-2]近年来全球范围内发病逐年增加,其恶性程度高、治疗难度大,以放射性核素177Lu标记奥曲肽(177Lu-DOTAD-Phe1-Tyr3-octreotate,177Lu-DOTA-TATE)进行肽受体放射性核素治疗(peptide-receptor radionuclide therapy,PRRT)[3-4]是治疗生长抑素受体高表达神经内分泌肿瘤的一项重要手段,并于2018年1月通过美国食品药品管理局(FDA)批准用于临床。
目前欧美国家已开展多年PRRT治疗,国内近期开始引入,如北京肿瘤医院就进行了177Lu-DOTA-TATE药物的研发并开展神经内分泌肿瘤治疗临床试验研究[5-7]。神经内分泌肿瘤PRRT治疗患者177Lu-DOTA-TATE的给药活度通常为每个疗程370~740 MBq,其在全身的分布主要为肝脏、肾脏、脾脏、膀胱等,因此PRRT对肿瘤进行治疗的同时也将对肾脏等敏感非靶组织产生较高的辐射损伤[8-9],而肾脏作为主要的危险器官有必要对其进行准确、可靠的吸收剂量估算,以了解PRRT治疗患者的安全性。
本文将借鉴国际原子能机构(International Atomic Energy Agency,IAEA)20号报告[10],同时参考医用内照射剂量(medical internal radiation dose,MIRD)11、16报告[11-12]等建立的标准人体模型和剂量计算方法,以吸收剂量作为评估指标,估算177Lu-DOTA-TATE治疗神经内分泌肿瘤患者肾脏吸收剂量,在肿瘤受到最大吸收剂量的同时保证危险器官肾脏所受吸收剂量不超过剂量限值(23 Gy)[10, 13-14]。
材料与方法 1、PRRT治疗患者经过临床适应证筛选合适的神经内分泌肿瘤PRRT治疗患者,符合条件者接受177Lu-DOTA-TATE注射,受检者均签署知情同意书,经过北京肿瘤医院伦理委员会批准(批号:2018YJZ04)。177Lu-DOTA-TATE通过自行制备获得,其中177Lu由德国ITG公司提供,前体DOTA-TATE购自法国ABX公司,标记产物通过高效液相色谱仪(1200,安捷伦,美国)测定放射化学纯度。
2、图像采集使用德国西门子公司Symbia T16双探头SPECT/CT进行平面全身成像,采用中能通用准直器,能峰208 keV(15%能窗),扫描速度15 cm/min,采集矩阵128×1 024。患者注射后采集5个时间点:0.5、3、20、44和116 h。
3、吸收剂量估算使用MIRD方法估算患者肾脏受到的吸收剂量[11-12],其计算公式为D=Ã×S,式中D为肾脏的吸收剂量,Ã为源器官累积活度(肾脏自身和全身作为源器官),S为吸收剂量因子。其计算过程主要包括如下4个步骤:①靶区的勾画:使用图像处理工作站(Syngo,西门子,德国)勾画平面图像的整个身体、肾脏区域和本底的前位/后位计数。②靶区活度的确定:患者注射前使用活度计(CRC-25R,CAPINTEC,美国)准确测量药物的初始活度,随即注射药物后开始第1个时间点(0.5 h)的图像采集,以第1个采集时间点的全身总计数(本底和散射校正)和注射初始活度的比值作为活度/计数校准因子,将靶器官计数转化成活度。③靶区累积活度计算:进行多个时间点的图像采集后,获得靶器官的活度-时间曲线(TAC),以单指数方程拟合TAC构建数学模型,从而获得药物在体内的有效半衰期,进一步进行曲线下面积积分获得靶区累积活度。④通过OLINDA/EXM 2.0(Hermes Medical Solutions,瑞士)软件,选择核素(177Lu)和标准参考人体模型(ICRP 89 Adult Model),同时输入源器官(肾脏和全身)的累积活度,估算肾脏的吸收剂量[15]。
4、数据处理数据以x±s表示,采用Excel 2016软件(微软,美国)进行相关数据处理。Origin 2019b(OriginLab,美国)进行单指数方程的拟合。
结果 1、177Lu-DOTA-TATE全身显像情况经筛选总共11名神经内分泌肿瘤患者进行了18例次177Lu-DOTA-TATE治疗,其中7名患者进行了1个疗程治疗,1名患者进行了2个疗程治疗,3名患者进行了3个疗程治疗,每个疗程的注射活度(3.8~7.3)×103 MBq,平均注射活度(6.3±0.9)×103 MBq。177Lu-DOTA-TATE注射到体内后主要通过肾脏和膀胱代谢,其全身平面显像及靶区勾画,见图 1。
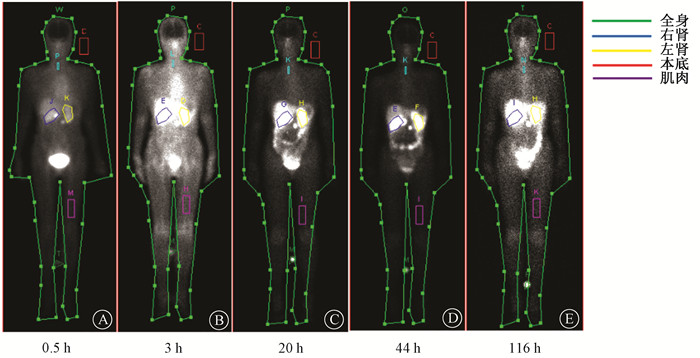
|
图 1 不同注射时间后177Lu-DOTA-TATE全身平面显像及靶区示意图 A. 0.5 h;B. 3 h;C. 20 h;D. 44 h;E. 116 h Figure 1 Diagrams of whole-body planar imaging and the ROIs obtained at different time after the administration of 177Lu-DOTA-TATE A. 0.5 h; B. 3 h; C. 3 h; D. 44 h; E. 116 h |
2、177Lu-DOTA-TATE全身和肾脏代谢情况
177Lu-DOTA-TATE治疗患者药物经过全身和肾脏代谢20 h后,其在体内的代谢情况符合指数衰变的趋势,如图 2为1例治疗患者典型的药物代谢过程。使用单指数方程拟合时间-活度曲线后计算累积活度和有效半衰期,所有治疗患者全身的有效半衰期(20.0~99.8)h,平均有效半衰期(57.3±21.4)h,相关系数R2 0.87~1.00(平均值0.95);肾脏的有效半衰期为(38.2~75.2)h,平均有效半衰期(53.1±12.5)h,相关系数R2 0.84~1.00(平均值0.97),见图 2。
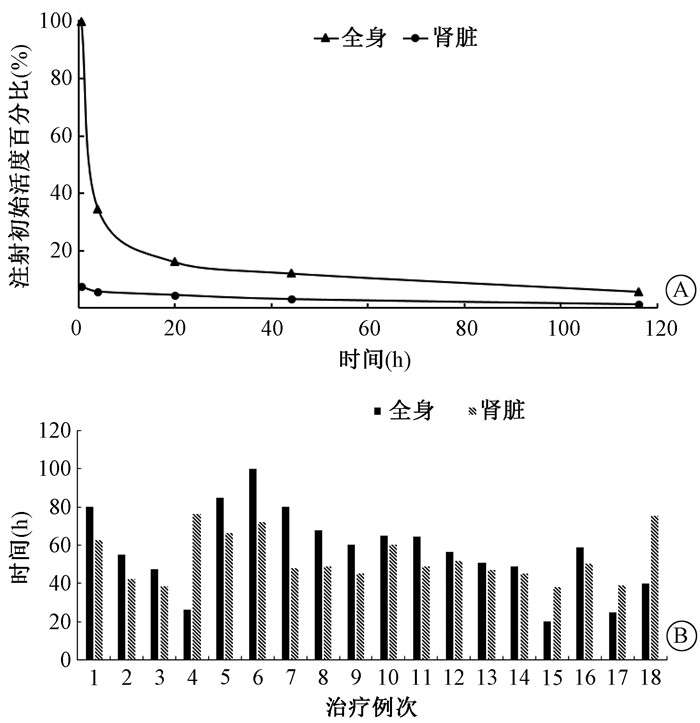
|
图 2 177Lu-DOTA-TATE全身和肾脏代谢情况Ⓐ 和有效半衰期Ⓑ Figure 2 An example of the distribution of 177Lu-DOTA-TATE in the whole body and kidneys Ⓐ and effective half-lives of all treatment cycles Ⓑ |
3、177Lu-DOTA-TATE肾脏吸收剂量
将获得的肾脏累积活度代入OLINDA/EXM 2.0软件,选取177Lu核素以及ICRP标准成人模型参数,计算得到177Lu-DOTA-TATE治疗患者肾脏受到的吸收剂量为(0.25~1.48)mGy/MBq,平均吸收剂量(0.90±0.31)mGy/MBq,见图 3。
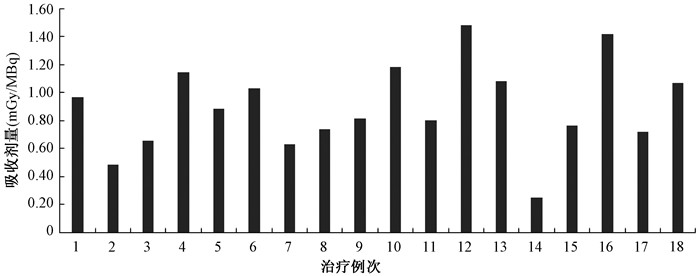
|
图 3 177Lu-DOTA-TATE治疗患者肾脏吸收剂量 Figure 3 Renal absorbed doses of 177Lu-DOTA-TATE in the treatments of patients |
同时对每名患者肾脏受到的累积吸收剂量进行计算,单次疗程肾脏受到的吸收剂量最小1.8 Gy,最大9.6 Gy,一名患者3次治疗后肾脏累积吸收剂量21.7 Gy,未有患者肾脏累积吸收剂量超过规定的剂量限值(23 Gy)[10],从而保证了每1名行177Lu-DOTA-TATE神经内分泌肿瘤治疗患者的辐射安全性,见图 4。
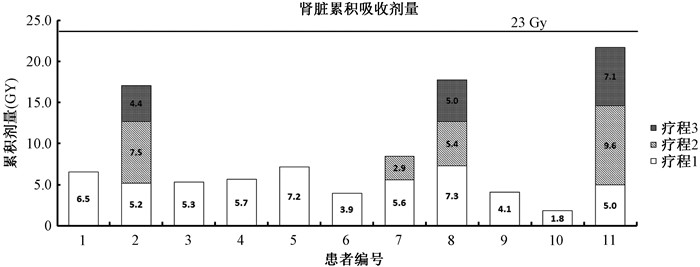
|
图 4 177Lu-DOTA-TATE治疗患者肾脏累积吸收剂量 Figure 4 Cumulative absorbed dose of 177Lu-DOTA-TATE of patients |
讨论
177Lu-DOTA-TATE神经内分泌肿瘤治疗的患者具有不同药物代谢和生理结构等,并且药物主要通过肾脏和膀胱代谢,肾脏受到的吸收剂量最大,也是最危险的器官之一,为了保障每名治疗患者的辐射安全,进行患者准确肾脏吸收剂量计算是十分必要的。本文通过精准的靶区勾画,优化的采集协议和方法,使用基于个体的剂量计算模型,获得了每名177Lu-DOTA-TATE神经内分泌肿瘤治疗患者的肾脏吸收剂量,并对其在全身和肾脏的代谢和半衰期情况进行分析,未有患者肾脏吸收剂量超过耐受限值。
同时将本文计算结果与其他文献报道的177Lu-DOTA-TATE神经内分泌肿瘤治疗患者肾脏受到的吸收剂量进行比较,文献[16-19]报道患者肾脏吸收剂量为(0.65 ~ 0.90)mGy/MBq,本文的研究结果(0.90±0.31)mGy/MBq与之总体平均值相当,但吸收剂量存在着个体化差异,这正是由于每名患者存在着诸如身高、体重、生理代谢等个体化差异情况,也进一步说明了对于177Lu-DOTA-TATE神经内分泌肿瘤治疗患者进行个体剂量估算的必要性。
本研究以传统的平面二维图像来勾画靶区,构建数学模型来估算患者肾脏受到的吸收剂量,而通过SPECT三维图像、蒙特卡罗方法以及体素因子法等方法进行吸收剂量估算是目前研究的热点[20-21],这些方法将更加准确地反映患者个体化吸收剂量,但是势必会增加患者的扫描时间,加重临床负担,估算过程也更为繁琐。如何进行简便的、临床实际操作可行的,以及更加精准的靶向吸收剂量的估算也将是本文下一步的研究重点。同时本研究中心也正在积累治疗患者的临床数据,对患者的临床治疗效果进行评估,下一步将进行肿瘤病灶的吸收剂量估算并与临床疗效相结合[22],以期指导个体化剂量靶向治疗。
利益冲突 无
志谢 本项目还受到北京大学肿瘤医院自然科学基金(2021-5)支助
作者贡献声明 王风负责设计实验和论文撰写;潘永祥负责数据采集;丁立新负责数据处理;朱华负责数据审核;于江媛负责分析和解释数据;杨志指导论文修改
| [1] |
Pavel M, de Herder WW. ENETS consensus guidelines for the standard of care in neuroendocrine tumors[J]. Neuroendo-crinology, 2017, 105(3): 193-195. DOI:10.1159/000457957 |
| [2] |
Garcia-Carbonero R, Rinke A, Valle JW, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasms. systemic therapy 2:chemotherapy[J]. Neuroendocrinology, 2017, 105(3): 281-294. DOI:10.1159/000473892 |
| [3] |
Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3)[J]. Endocr Relat Cancer, 2020, 27(3): R67-67R77. DOI:10.1530/ERC-19-0400 |
| [4] |
Thang SP, Lung MS, Kong G, et al. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3(G3) neuroendocrine neoplasia (NEN)-a single-institution retrospective analysis[J]. Eur J Nucl Med Mol Imaging, 2018, 45(2): 262-277. DOI:10.1007/s00259-017-3821-2 |
| [5] |
Yu J, Li N, Li J, et al. The Correlation Between 168Ga DOTA-TATE PET/CT and cell proliferation in patients with GEP-NENs[J]. Mol Imaging Biol, 2019, 21(5): 984-990. DOI:10.1007/s11307-019-01328-3 |
| [6] |
朱华, 于江媛, 李囡, 等. 68Ga-DOTA-TATE的制备及在神经内分泌肿瘤显像中的应用[J]. 中华核医学与分子影像杂志, 2015, 12(35): 487-491. Zhu H, Yu JY, Li N, et al. Preparation of 68Ga-DOTA-TATE and its clinical trial in neuroendocrine tumor[J]. Chin J Nucl Med Mol Imaging, 2015, 12(35): 487-491. DOI:10.3760/cma.j.issn.2095-2848.2015.06.016 |
| [7] |
Liu F, Zhu H, Yu J, et al. 68Ga/177Lu-labeled DOTA-TATE shows similar imaging and biodistribution in neuroendocrine tumor model[J]. Tumour Biol, 2017, 39(6): 1010428317705519. DOI:10.1177/1010428317705519 |
| [8] |
Del Prete M, Arsenault F, Saighi N, et al. Accuracy and reproducibility of simplified QSPECT dosimetry for personalized 177Lu-octreotate PRRT[J]. EJNMMI Phys, 2018, 5(1): 25. DOI:10.1186/s40658-018-0224-9 |
| [9] |
Sundlöv A, Sjögreen-Gleisner K, Svensson J, et al. Individualised 177Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry[J]. Eur J Nucl Med Mol Imaging, 2017, 44(9): 1480-1489. DOI:10.1007/s00259-017-3678-4 |
| [10] |
International Atomic Energy Agency. IAEA report No. 20 Practical guidance on peptide receptor radionuclide therapy (PRRNT) for neuroendocrine tumors[R]. Vienna: IAEA, 2013.
|
| [11] |
Snyder WS, Ford MR, Warner GG, et al. MIRD pamphlet No 11: "S" absorbed dose per unit cumulated activity for selected radionuclides and organs[R]. New York: Society of Nuclear Medicine, 1975.
|
| [12] |
Siegel JA, Thomas SR, Stubbs JB, et al. Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates: MIRD Pamphlet No.16[J]. J Nucl Med, 1999, 40(suppl): S37-S61. |
| [13] |
Bodei L, Mueller-Brand J, Baum RP, et al. Erratum to: The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy(PRRNT) in neuroendocrine tumours[J]. Eur J Nucl Med Mol Imaging, 2014, 41: 584. DOI:10.1007/s00259-013-2454-3 |
| [14] |
Cremonesi M, Ferrari M, Bodei L, et al. Dosimetry in peptide radionuclide receptor therapy: a review[J]. J Nucl Med, 2006, 47(9): 1467-1475. DOI:10.1016/j.cca.2008.08.012 |
| [15] |
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine[J]. J Nucl Med, 2005, 46(6): 1023-1027. DOI:10.1016/S0150-9861(05)83140-2 |
| [16] |
Kwekkeboom DJ, Bakker WH, Kooij PP, et al. [177Lu-DOTAOTyr3] octreotate: comparison with[111In-DTPAo] octreotide in patients[J]. Eur J Nucl Med, 2001, 28(9): 1319-1325. DOI:10.1007/s002590100574 |
| [17] |
Cremonesi M, Ferrari M, Bodei L, et al. Dosimetry in patients undergoing 177Lu-DOTATATE therapy with indications for 90Y-DOTATATE[J]. Eur J Nucl Med Mol Imaging, 2006, 33: S102. DOI:10.1007/s00259-006-0151-1 |
| [18] |
Wehrmann C, Senftleben S, Zachert C, et al. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC[J]. Cancer Biother Radiopharm, 2007, 22(3): 406-416. DOI:10.1089/cbr.2006.325 |
| [19] |
Sandstr öm M, Garske U, Granberg D, et al. Individualized dosimetry in patients undergoing therapy with 177Lu-DOTA-D-Phe (1)-Tyr (3)-octreotate[J]. Eur J Nucl Med Mol Imaging, 2010, 37(2): 212-225. DOI:10.1007/s00259-009-1216-8 |
| [20] |
Hou X, Zhao W, Beauregard JM, et al. Personalized kidney dosimetry in 177Lu-octreotate treatment of neuroendocrine tumours: a comparison of kidney dosimetry estimates based on a whole organ and small volume segmentations[J]. Phys Med Biol, 2019, 64(17): 175004. DOI:10.1088/1361-6560/ab32a1 |
| [21] |
Jackson PA, Beauregard JM, Hofman MS, et al. An automated voxelized dosimetry tool for radionuclide therapy based on serial quantitative SPECT/CT imaging[J]. Med Phys, 2013, 40(11): 112503. DOI:10.1118/1.4824318 |
| [22] |
Sundlöv A, Sj green-Gleisner K, Svensson J, et al. Individualised 177Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry[J]. Eur J Nucl Med Mol Imaging, 2017, 44(9): 1480-1489. DOI:10.1007/s00259-017-3678-4 |
 2021, Vol. 41
2021, Vol. 41


