结外鼻型NK/T细胞淋巴瘤(extranodal NK/T cell lymphoma, nasal type, ENKTCL)是一种侵袭性较强且异质性较大的淋巴瘤,该病在全世界范围内罕见,主要分布在东亚和拉丁美洲[1-4]。结外鼻型NK/T细胞淋巴瘤与EB病毒感染相关;在临床上以年轻、男性和早期患者多见,初诊Ⅰ~Ⅱ期患者占70%~90%;肿瘤常见于上呼吸消化道,尤以原发鼻腔最为多见[1, 5-6]。因发病率低,目前研究多为回顾性和小样本Ⅰ/Ⅱ期临床研究,针对Ⅰ~Ⅱ期NK/T细胞淋巴瘤,目前尚无标准治疗方案。既往研究结果显示,结外鼻型NK/T细胞淋巴瘤对蒽环类为基础的传统化疗抗拒,主要因为其高表达多药耐药P蛋白[7]。近些年,非蒽环类为基础的化疗方案广泛应用于早期及晚期NK/T细胞淋巴瘤患者,如异环磷酰胺、甲氨蝶呤和依托泊苷等。以上述药物为基础的一线化疗方案可达到77%~80%完全缓解率(complete response, CR)和81%~83% 的总有效率(overall response rate, ORR)[8-10]。NK/T细胞淋巴瘤的肿瘤细胞缺乏门冬酰胺合成酶,而左旋门冬酰胺酶可以降解血清中的天门冬酰胺,抑制肿瘤细胞的蛋白质合成,发挥抗肿瘤作用。因此,基于左旋门冬酰胺酶(或培门冬酶)的化疗方案开始得到广泛的应用[11-14]。NK/T细胞淋巴瘤对放疗敏感,放疗后可以达到较高的完全缓解率,因此,放疗在早期NK/T细胞淋巴瘤的治疗中发挥至关重要的作用[15-16]。
目前对于最佳的放化疗顺序及化疗方案仍待研究,常用方案有同步放化疗[8, 17]、序贯放化疗[18-20]和“三明治”疗法[21-23]。目前尚无头对头(head-to-head)研究证实最佳放化疗次序,因此国内外各单位间差异较大。基于以上研究结果,本研究分析本院收治的早期结外鼻型NK/T细胞淋巴瘤数据,探讨放化综合治疗的价值以及不良反应情况。
资料与方法 1、研究人群回顾性分析2003年4月至2019年9月北京大学肿瘤医院放疗科收治的结外鼻型NK/T细胞淋巴瘤患者。入组标准包括:①根据世界卫生组织(WHO)淋巴瘤分类标准,明确病理诊断为结外鼻型NK/T细胞淋巴瘤。②Ann Arbor分期为ⅠE~ⅡE期。③在本科室接受放射治疗,临床资料和生存随访资料完整。符合入组标准患者共174例。
2、分期检查及预后模型所有患者治疗前均完善体格检查、血常规、肝肾功能、血清乳酸脱氢酶(serum lactate dehydrogenase,LDH)、头颈部CT和(或)MRI、胸腹盆CT及骨髓检查,其中有98例患者治疗前行PET-CT检查。治疗前后EB病毒DNA拷贝数检查未做强制要求。全组患者均采用Ann Arbor分期,并依据列线图调整风险指数(nomogram-revised risk index, NRI)预后模型进行评分。原发肿瘤侵犯(primary tumor invasion,PTI)定义为原发肿瘤侵犯临近组织结构或器官,如肿瘤位于鼻腔侵犯副鼻窦和(或)鼻咽;或原发肿瘤连续侵犯多个部位而不考虑肿瘤分期或原发部位[24]。NRI根据列线图模型中5个因素,即年龄>60岁、东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)评分≥2分、LDH升高、分期(Ⅰ期vs.Ⅱ期vs.Ⅲ/Ⅳ期)、PTI,分成不同危险组[25]。
3、治疗方法共有50例患者(28.7%)接受原发灶切除或部分切除。2例患者接受单纯放疗,5例患者接受放疗序贯化疗,29例患者接受化疗序贯放疗,28例患者接受化疗+放疗+化疗,12例患者接受化疗+同步放化疗,97例患者接受化疗+同步放化疗+化疗,1例患者接受同步放化疗。
放疗采用直线加速器,其中5例患者采用二维放疗技术,169例患者采用调强放射治疗(intensity modulated radiotherapy, IMRT)或容积旋转调强放射治疗(volumetric modulated arc therapy, VMAT)技术。原发灶及受侵区域中位放疗剂量为54 Gy(18~66 Gy),其中19例(10.9%)放疗剂量 < 50 Gy。进一步分析未能接受50 Gy放疗原因,8例患者因严重不良反应中断治疗,11例患者因各种原因处方剂量未达50 Gy。放疗采用扩大受累部位照射,GTV包括原发灶及受侵淋巴结,CTV包括受累的整个器官和邻近结构,若颈部淋巴结受侵,需照射全颈部。颈部预防照射由临床医生评估决定。
化疗采用CHOP或CHOP类似方案10例,左旋门冬酰胺酶或培门冬酶为基础的方案有162例患者。放疗前化疗中位周期数为2(1~7),中位总化疗周期数为6(2~9)。
4、生存分析根据2014年Lugano分类来评估近期疗效[26]。完全缓解(complete response, CR)定义为所有影像学可见病灶完全消失;部分缓解(partial response, PR)定义为肿瘤部分退缩且无新发病灶;肿瘤进展(progression disease, PD)定义为新发病灶或原有肿瘤增长>50%,;稳定病灶(stable disease, SD)定义为肿瘤变化介于CR(PR)与PD之间。总生存率(overall survival, OS)定义为从初始治疗开始到任何原因死亡或末次随访时间,无进展生存率(progression free survival, PFS)定义为从初始治疗到首次进展、复发、末次随访或任何原因死亡的时间,局部区域控制率(local regional control, LRC)定义为从初始治疗到首次出现局部或区域复发、末次随访时间或任何原因死亡时间。早期不良反应按照不良事件通用术语评价标准5.0(common terminology criteria for adverse events,CTCAE 5.0)进行分级,晚期不良反应按照美国肿瘤放射治疗协作组(Radiation Therapy Oncology Group,RTOG)晚期放射损伤标准进行分级。
4、统计学处理应用SPSS 19.0软件进行统计分析。采用Kaplan-Meier法进行生存分析,Log-rank检验行单因素分析,COX回归模型行多因素分析。P < 0.05为差异有统计学意义。
结果 1、一般临床特征全组共有174例患者,其中男女比为2.05 ∶1,中位年龄为41岁(15~85岁),原发部位中有140例(80.5%)为鼻腔原发,其余原发部位为:鼻咽17例、韦氏环11例、喉2例、口腔2例、皮肤1例、淋巴结1例。其余临床特征详见表 1。
|
|
表 1 全组患者一般临床资料特点 Table 1 General clinical characteristics of all patients |
2、生存分析结果
全组客观缓解率(ORR)为94.2%(164/174),其中CR患者153例(87.9%),PR患者11例,PD患者5例,未评价5例。中位随访时间为65个月(6~176个月),全组患者5年OS为87.3%,5年PFS为83.1%,5年LRC为91.9%。
进一步行单因素分析发现,高龄、B症状、原发部位非鼻腔为OS不良预后因素,高龄是PFS不良预后因素,没有发现LRC的不良预后因素(表 2)。总生存结果曲线及不同Ann Arbor分期和NRI状态患者的生存曲线见图 1。Ann Arbor分期Ⅰ/Ⅱ期患者的OS和PFS相似,而NRI分组为中危和中高危患者预后差于低危和低中危组患者。
|
|
表 2 全组患者单因素预后分析结果 Table 2 Log\|rank test results of all patients |
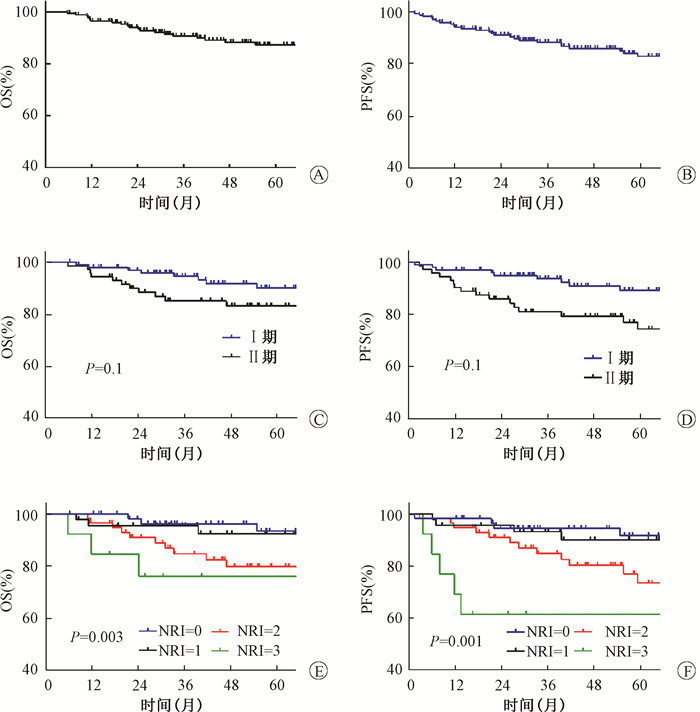
|
注:OS.总生存;PFS.无进展生存;NRI.列线图调整风险指数 图 1 早期结外鼻型NK/T细胞淋巴瘤总体OS(A)、PFS(B)及不同Ann Arbor分期(C、D) 及NRI评分(E、F)组OS及PFS比较 Figure 1 Survival curves of overall survival and progression free survival in all patients(A and B), different Ann Arbor(C and D) and NRI groups(E and F) |
多因素分析结果显示,高龄、B症状及Ann Arbor分期Ⅱ期是OS的独立预后不良因素,而高龄和Ann Arbor分期Ⅱ期是PFS的独立预后不良因素(表 3)。进一步分析发现,放疗剂量≥50 Gy较低剂量组可显著提高PFS,两组5年PFS分别为83.5%和76.5%(HR 0.374,95%CI 0.169~0.826,P=0.015)。不同放疗剂量组5年OS分别为88.7%和77.4%(HR 0.471,95%CI 0.182~1.220,P=0.118),差异无统计学意义。
|
|
表 3 全组患者多因素预后分析结果 Table 3 COX regression results of all patients |
4、复发模式
截至末次随访,全组有32例患者复发进展,其中12例(6.9%)患者出现局部复发,6例(3.4%)患者出现区域淋巴结复发,13例患者(7.5%)出现远地结外器官或远地淋巴结失败。共25例患者死亡,其中23例因肿瘤去世,1例因第二原发肿瘤去世,1例因心脏病去世。
5、近期及远期不良反应放化疗期间最常见的不良反应为骨髓抑制和口腔黏膜炎,≥3级骨髓抑制占62.1%,以白细胞和中性粒细胞减低为主,口腔黏膜炎、皮肤反应、肝损伤及胃肠道反应大多为1~2级(表 4)。在治疗期间,15例患者放疗中断或停止治疗,放疗中断天数为2~70 d,其中有2例患者放疗中断超过30 d。中断原因主要为中重度黏膜炎、重度疼痛等。9例因3~4级不良反应导致化疗推迟或中断。无治疗相关死亡。对于晚期不良反应,因长期不良事件随访资料记录不完整,不能做详细分析。随访期间未发现3级及以上不良反应。
|
|
表 4 全组患者放化疗期间急性不良反应(例) Table 4 Acute toxicities of radiotherapy and chemotherapy in all patients |
讨论
本研究回顾性分析了174例接受放疗的早期结外鼻型NK/T细胞淋巴瘤患者资料,全组患者5年OS为87.3%,5年PFS为83.1%,5年LRC为91.9%。亚组分析显示,放疗剂量≥50 Gy较低剂量组可显著提高总PFS。整体不良反应可耐受。
放疗在早期NK/T细胞淋巴瘤的治疗中发挥着不可替代的作用,Li等[15]奠定了蒽环类化疗年代放疗的基础。美国国家癌症数据库(National Cancer Database, NCDB)分析结果也表明,单纯化疗或放疗剂量不足(< 50 Gy)与总生存差有关[27]。我国多中心数据库结果显示,高剂量放疗(≥50 Gy)显著改善5年LRC(85% vs. 73%,P < 0.001)、PFS(61% vs. 50%,P=0.004)和OS(70% vs. 58%,P=0.04),且获益呈线性相关[28]。本研究中,所有患者均接受放射治疗,其中19例(10.9%)放疗剂量 < 50 Gy。放疗剂量≥50 Gy较低剂量组可显著提高总PFS,两组5年PFS分别为83.5%和76.5%(P=0.015),两组5年OS分别为88.7%和77.4%,但差异无统计学意义,考虑可能与样本量较少有关。
既往对于早期NK/T细胞淋巴瘤放化疗的Ⅱ研究中,因样本量较小,生存结果差异较大,5年OS为66%~82.1%,5年PFS为60%~79.4%[11, 20, 29-31]。一项针对国内多中心回顾性大样本数据分析显示,针对早期结外鼻型NK/T细胞淋巴瘤,尤其是具有高危因素的患者,以左旋门冬酰胺酶或培门冬酶为基础的放化综合治疗方案较单纯化疗或放疗可显著改善预后[14]。本研究中,93.1%患者接受左旋门冬酰胺酶或培门冬酶为基础的化疗方案,且91.2%患者接受≥50 Gy放疗,全组患者5年OS为87.3%,5年PFS为83.1%,与既往研究结果类似。
既往研究中以左旋门冬酰胺酶或培门冬酶为基础的Ⅱ期和回顾性研究结果显示,ORR为85.7%~94.3%,CR率为57.1%~93.3%,≥3级血液学不良反应为16.7%~43%,≥3级口腔黏膜炎10%~33%[8, 11, 32-35]。本研究中,全组ORR为94.2%(164/174),其中CR患者153例(87.9%),显示出较好的近期疗效。本研究中,≥3级骨髓抑制占62.1%,发生比例较高,本院近些年针对高危患者预防性应用长效升白针减少了3/4度中性粒细胞减少及感染风险。本研究中≥3级口腔黏膜炎为10.9%,发生比例较低,与本组大多数患者采用IMRT/VMAT照射技术有关,同时与积极应用肠内、肠外营养支持治疗有关,整体上患者耐受性较好。
在NK/T细胞淋巴瘤中,比较重要的几个预后模型分别为IPI、KPI、PINK和NRI[25, 36-38],在这几个模型中,预后因素为年龄、B症状、LDH、ECOG评分、分期、结外侵犯、原发肿瘤部位等,本研究多因素分析结果显示,高龄、B症状及Ⅱ期是OS的独立预后不良因素,而高龄和Ⅱ期是PFS的独立预后不良因素,与既往研究相似。
基于以上结果,本研究显示对于早期结外鼻型NK/T细胞淋巴瘤,以左旋门冬酰胺酶或培门冬酶为基础的化疗联合50 Gy以上的常规分割剂量放疗,可以达到国内外文献报道的相似治疗效果,不良反应可耐受。
利益冲突 本研究由署名作者按以下贡献声明独立开展,不涉及任何利益冲突,排名无争议
作者贡献声明 刘伟欣负责数据分析和论文撰写;赵丹、徐晓龙和黄州负责数据收集整理,协助撰写论文;肖绍文、郑宝敏参与统计分析,指导论文书写;林宁晶、宋玉琴和王维虎参与研究设计及论文审阅;孙艳负责研究设计,指导论文修改
| [1] |
Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project[J]. Blood, 2009, 113(17): 3931-3937. DOI:10.1182/blood-2008-10-185256 |
| [2] |
Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in southwest China: analysis of 6, 382 cases using WHO classification in a single institution[J]. Diagn Pathol, 2011, 6: 77. DOI:10.1186/1746-1596-6-77 |
| [3] |
Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China: analysis of 4, 638 cases according to the World Health Organization classification[J]. Am J Clin Pathol, 2012, 138(3): 429-434. DOI:10.1309/AJCP7YLTQPUSDQ5C |
| [4] |
Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States[J]. J Clin Oncol, 2016, 34(9): 963-971. DOI:10.1200/JCO.2015.63.5540 |
| [5] |
Chen SY, Yang Y, Qi SN, et al. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making[J]. Leukemia, 2021, 35(1): 130-142. DOI:10.1038/s41375-020-0791-3 |
| [6] |
Kim TM, Lee SY, Jeon YK, et al. Clinical heterogeneity of extranodal NK/T-cell lymphoma, nasal type: a national survey of the Korean Cancer Study Groxup[J]. Ann Oncol, 2008, 19(8): 1477-1484. DOI:10.1093/annonc/mdn147 |
| [7] |
Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells[J]. Cancer, 1995, 76(11): 2351-2356. DOI:10.1002/1097-0142(19951201)76:11<2351::aid-cncr2820761125>3.0.co;2-1 |
| [8] |
Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study[J]. J Clin Oncol, 2009, 27(35): 6027-6032. DOI:10.1200/JCO.2009.23.8592 |
| [9] |
Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL)[J]. Ann Hematol, 2015, 94(3): 437-444. DOI:10.1007/s00277-014-2228-4 |
| [10] |
Nomura E, Isoda KI, Yamanaka K, et al. Extra nodal NK/T-cell lymphoma nasal type that responded to DeVIC combination chemotherapy[J]. J Dermatol, 2005, 32(3): 204-209. DOI:10.1111/j.1346-8138.2005.tb00746.x |
| [11] |
Kim SJ, Yang DH, Kim JS, et al. Concurrent chemoradiotherapy followed by L-asparaginase-containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08-01 phase II study[J]. Ann Hematol, 2014, 93(11): 1895-1901. DOI:10.1007/s00277-014-2137-6 |
| [12] |
Lin N, Song Y, Zheng W, et al. A prospective phase II study of L-asparaginase-CHOP plus radiation in newly diagnosed extranodal NK/T-cell lymphoma, nasal type[J]. J Hematol Oncol, 2013, 6: 44. DOI:10.1186/1756-8722-6-44 |
| [13] |
Wang L, Wang WD, Xia ZJ, et al. Combination of gemcitabine, L-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up[J]. Med Oncol, 2014, 31(3): 860. DOI:10.1007/s12032-014-0860-4 |
| [14] |
Qi SN, Yang Y, Song YQ, et al. First-line non-anthracycline-based chemotherapy for extranodal nasal-type NK/T-cell lymphoma: a retrospective analysis from the CLCG[J]. Blood Adv, 2020, 4(13): 3141-3153. DOI:10.1182/bloodadvances.2020001852 |
| [15] |
Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma[J]. J Clin Oncol, 2006, 24(1): 181-189. DOI:10.1200/JCO.2005.03.2573 |
| [16] |
Huang MJ, Jiang Y, Liu WP, et al. Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract[J]. Int J Radiat Oncol Biol Phys, 2008, 70(1): 166-174. DOI:10.1016/j.ijrobp.2007.05.073 |
| [17] |
Wang H, Wang L, Li C, et al. Pegaspargase combined with concurrent radiotherapy for early-stage extranodal natural killer/T-cell lymphoma, nasal type: a two-center phase II study[J]. Oncologist, 2020, 25(11): e1725-1725e1731. DOI:10.1634/theoncologist.2020-0144 |
| [18] |
Huang Y, Yang J, Liu P, et al. Intensity-modulated radiation therapy followed by GDP chemotherapy for newly diagnosed stage I/II extranodal natural killer/T cell lymphoma, nasal type[J]. Ann Hematol, 2017, 96(9): 1477-1483. DOI:10.1007/s00277-017-3046-2 |
| [19] |
Kwong YL, Kim SJ, Tse E, et al. Sequential chemotherapy/radiotherapy was comparable with concurrent chemoradiotherapy for stage I/II NK/T-cell lymphoma[J]. Ann Oncol, 2018, 29(1): 256-263. DOI:10.1093/annonc/mdx684 |
| [20] |
Kim TM, Kim DW, Kang YK, et al. A phase II study of ifosfamide, methotrexate, etoposide, and prednisolone for previously untreated stage I/II extranodal natural killer/T-cell lymphoma, nasal type: a multicenter trial of the Korean Cancer Study Group[J]. Oncologist, 2014, 19(11): 1129-1130. DOI:10.1634/theoncologist.2014-0305 |
| [21] |
Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of "sandwich" L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma[J]. Cancer, 2012, 118(13): 3294-3301. DOI:10.1002/cncr.26629 |
| [22] |
Jiang M, Zhang L, Xie L, et al. A phase II prospective study of the "Sandwich" protocol, L-asparaginase, cisplatin, dexamethasone and etoposide chemotherapy combined with concurrent radiation and cisplatin, in newly diagnosed, I/II stage, nasal type, extranodal natural killer/T-cell lymphoma[J]. Oncotarget, 2017, 8(30): 50155-50163. DOI:10.18632/oncotarget.16334 |
| [23] |
Wang HY, Niu SQ, Yang YY, et al. Promising clinical outcomes of sequential and "Sandwich" chemotherapy and extended involved-field intensity-modulated radiotherapy in patients with stage IE/IIE extranodal natural killer/T-cell lymphoma[J]. Cancer Med, 2018, 7(12): 5863-5869. DOI:10.1002/cam4.1755 |
| [24] |
Qi SN, Xu LM, Yuan ZY, et al. Effect of primary tumor invasion on treatment and survival in extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: a multicenter study from the China Lymphoma Collaborative Group (CLCG)[J]. Leuk Lymphoma, 2019, 60(11): 2669-2678. DOI:10.1080/10428194.2019.1602265 |
| [25] |
Chen SY, Yang Y, Qi SN, et al. Validation of nomogram-revised risk index and comparison with other models for extranodal nasal-type NK/T-cell lymphoma in the modern chemotherapy era: indication for prognostication and clinical decision-making[J]. Leukemia, 2021, 35(1): 130-142. DOI:10.1038/s41375-020-0791-3 |
| [26] |
Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. DOI:10.1200/JCO.2013.54.8800 |
| [27] |
Vargo JA, Patel A, Glaser SM, et al. The impact of the omission or inadequate dosing of radiotherapy in extranodal natural killer T-cell lymphoma, nasal type, in the United States[J]. Cancer, 2017, 123(16): 3176-3185. DOI:10.1002/cncr.30697 |
| [28] |
Yang Y, Cao JZ, Lan SM, et al. Association of improved locoregional control with prolonged survival in early-stage extranodal nasal-type natural killer/T-cell lymphoma[J]. JAMA Oncol, 2017, 3(1): 83-91. DOI:10.1001/jamaoncol.2016.5094 |
| [29] |
Tsai HJ, Lin SF, Chen CC, et al. Long-term results of a phase II trial with frontline concurrent chemoradiotherapy followed by consolidation chemotherapy for localized nasal natural killer/T-cell lymphoma[J]. Eur J Haematol, 2015, 94(2): 130-137. DOI:10.1111/ejh.12405 |
| [30] |
Xu PP, Xiong J, Cheng S, et al. A phase II study of methotrexate, etoposide, dexamethasone and pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage IE to IIE extranodal natural-killer/T-cell lymphoma, nasal-type[J]. EBioMedicine, 2017, 25: 41-49. DOI:10.1016/j.ebiom.2017.10.011 |
| [31] |
Qi F, Wang WH, He XH, et al. Phase 2 study of first-line intensity modulated radiation therapy followed by gemcitabine, dexamethasone, and cisplatin for high-risk, early stage extranodal nasal-type NK/T-cell lymphoma: the GREEN study[J]. Int J Radiat Oncol Biol Phys, 2018, 102(1): 61-70. DOI:10.1016/j.ijrobp.2018.05.046 |
| [32] |
Huang L, Yuan B, Wu H, et al. Comparative study of L-asparaginase-based LOP regimen over CHOP regimen before radiotherapy for stage IIE extranodal nasal type NK/T cell lymphoma: A study of 2 centers[J]. Clin Lymphoma Myeloma Leuk, 2017, 17(3): 152-158. DOI:10.1016/j.clml.2016.12.003 |
| [33] |
Wei W, Wu P, Li L, et al. Effectiveness of pegaspargase, gemcitabine, and oxaliplatin (P-GEMOX) chemotherapy combined with radiotherapy in newly diagnosed, stage IE to IIE, nasal-type, extranodal natural killer/T-cell lymphoma[J]. Hematology, 2017, 22(6): 320-329. DOI:10.1080/10245332.2016.1264163 |
| [34] |
Zhu F, Liu T, Pan H, et al. Long-term outcomes of upfront concurrent chemoradiotherapy followed by P-GDP regimen in newly diagnosed early stage extranodal nasal-type NK/T cell lymphoma: A prospective single-center phase II study[J]. Medicine (Baltimore), 2020, 99(33): e21705. DOI:10.1097/MD.0000000000021705 |
| [35] |
Hu Y, Chen M, Song Y, et al. Study of L-asparaginase, vincristine, and dexamethasone combined with intensity-modulated radiation therapy in early-stage nasal NK/T-cell lymphoma[J]. Am J Clin Oncol, 2020, 43(4): 257-262. DOI:10.1097/COC.0000000000000647 |
| [36] |
International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma[J]. N Engl J Med, 1993, 329(14): 987-994. DOI:10.1056/NEJM199309303291402 |
| [37] |
Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis[J]. Lancet Oncol, 2016, 17(3): 389-400. DOI:10.1016/S1470-2045(15)00533-1 |
| [38] |
Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study[J]. DOI: 10.1200/JCO.2005.04.1384.
|
 2021, Vol. 41
2021, Vol. 41


