2. 北京大学肿瘤医院放射肿瘤科 100142
2. Department of Radiation Oncology, Peking University Cancer Hospital, Beijing 100142, China
放射性肠炎是宫颈癌及子宫内膜癌患者放射治疗期间常见的并发症,在妇科肿瘤的盆腔放射治疗中,有75%~89%的患者在治疗后发生了排便习惯的改变,其中49%~79%的患者表示影响了其生活质量,并且有23%~26%的患者出现了直肠出血症状[1]。目前尚无有效方法在保证不减少放射剂量的同时减轻或控制此症状[2-4]。
人体的肠道存在多达1×1014种微生物,总质量约2 kg[5]。肠道微生物与人体的生命活动有着密切联系,包括调节肠道内分泌功能、免疫功能和神经信号以及提供生物源能量、合成神经递质及代谢胆汁等[5-7]。另外,肠道微生物也与许多疾病甚至癌症的发病及治疗都有密切关系[8-9]。一些动物实验[10-12]及临床研究[13-16]表明,盆腔辐射可以改变肠道微生物群,而肠道微生物群的特征也会影响辐射敏感性,导致患者出现不同严重程度的放射性肠炎。
本研究使用16S rRNA高通量测序技术,探索宫颈癌及子宫内膜癌患者的肠道微生物与放疗期间放射性肠炎严重程度的关系。
资料与方法 1、排除标准既往接受过放射治疗;最近1个月内服用过抗生素;术后不超过1个月;肠道疾病史;不良排便习惯;其他肿瘤史;年龄>70岁。
2、患者资料在2020年2月至10月在北京大学人民医院及北京大学肿瘤医院招募37例宫颈癌或子宫内膜癌拟行放射治疗的患者入组,年龄38~68岁,中位年龄53岁,患者临床资料见表 1。
|
|
表 1 患者基本信息 Table 1 Patient characteristics |
3、样本收集及症状记录
所有入组对象在接受放疗前1周内及接受放疗15次左右(25.2~30.6 Gy /14~17次,1.8 Gy/次,中位剂量27 Gy)分别收集1次粪便样本,即刻速冻于液氮中并转移至-80℃冰箱保存,共收集到67份有效样本。本研究符合赫尔辛基宣言,并经过北京大学人民医院伦理委员会批准(2020PHB155-01)。
所有入组对象从接受放射治疗开始,根据常见不良反应事件评价标准(CTCAE)5.0分级的腹泻与直肠炎标准每周记录肠道症状[17]。将第2次取样前腹泻、直肠炎任意症状2级及以上的患者归为高症状级别组(HG),1级或无腹泻、直肠炎患者归为低症状级别组(LG)。19例为HG组,18例为LG组。按治疗方式分为根治性放疗(RR)组和术后放疗(PR)组。在整个放疗过程中没有患者出现4级与5级腹泻或直肠炎。
4、16S rRNA测序采用QIAamp Power Fecal DNA Kit对所有样本进行DNA提取,同时采用Nanodrop对DNA进行定量,并通过1.2%琼脂糖凝胶电泳检测DNA提取质量。对微生物核糖体RNA的V3/V4基因片段进行PCR扩增。对扩增产物进行磁珠纯化回收并荧光定量。最后,采用Illumina MiSeq/NovaSeq平台进行双端测序。用DADA2方法进行去引物、质量过滤、去噪、拼接和去嵌合体等步骤[18]。使用DADA2质量控制后产生的每个去重的序列称为ASVs (amplicon sequence variants),相当于以100%相似度聚类,效果优于传统OUT聚类。后续分析基于ASVs。
5、统计学处理对α多样性的评估,以Chao1指数表征丰富度,Shannon指数表征多样性。对β多样性的评估,采用非加权UniFrac距离(nonmetric multidimensional scaling) NMDS分析,stress<0.2表明两组差异分析可靠。物种差异分析及标志物种的寻找,采用LDA Effect Size (LEfSe)分析[19]及MetagenomeSeq分析[20],P<0.05为差异有统计学意义。
结果 1、肠道微生物群特征与放射性肠炎的相关性分析LG组和HG组的肠道微生物组成和丰度差异有统计学意义。放疗前LG组的α多样性高于HG组(Chao1:P=0.011,Shannon:P=0.009,图 1A)。两组的β多样性也有较大差异(stress=0.141,图 1B)。在经过3周放疗后,LG组的α多样性仍高于HG组(Chao1:P=0.042,Shannon:P=0.06,图 1C),两组的β多样性差异也有所降低(stress=0.161,图 1D)。通过LEfSe分析发现,放疗前样本LG组中Barnesiellaceae科、Barnesiella属和Paraprevotella属等丰度较高,HG组中Collinsella属、Ruminococcus属和Erysipelotrichaceae科Clostridium属等丰度较高(图 2 A、B)。两组之间的微生物差别在经过3周放疗之后也有所改变,LG组丰度更高的物种有Coriobacteriales目、Coriobacteriaceae科和Coriobacteriia纲等,而HG组相对丰度更高的物种为Eubacterium属、Erysipelotrichaceae科Clostridium属和Lautropia属等(图 2C、D)。为了进一步找出两组差别较大的物种,通过MetagenomeSeq分析在种的水平上发现,放疗前HG组中Ruminococcus gnavus高于LG组(P=0.002,图 3)。提示Ruminococcus gnavus有作为放射治疗前预测放射性肠炎严重程度的标志物种的可能。另外,通过MetagenomeSeq分别分析了LG组与HG组两组放疗前后的肠道微生物差别。两组放疗前较放疗后显著上调的物种见表 2。而放疗后较放疗前显著上调的物种两组均未发现。
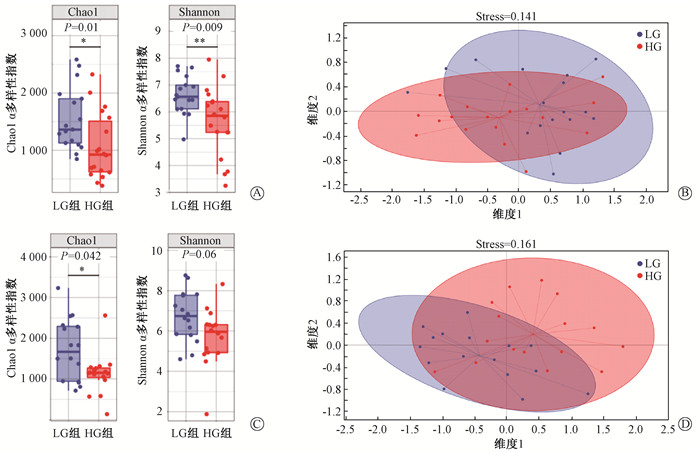
|
注:Stress<0.2说明结果可靠 图 1 低症状级别组(LG)与高症状级别组(HG)微生物多样性分析 A、C.放疗前和放疗后样本LG组与HG组的α多样性对比箱线图;B、D.放疗前和放疗后LG组与HG组基于Unweighted-Unifrac的NMDS分析 Figure 1 Microbial diversity analysis of the LG and the HG groups A, C. Box plots of α diversity comparison between the LG and HG groups before and after radiotherapy; B, D. NMDS analysis based on Unweighted Unifrac of the LG and HG groups before and after radiotherapy |
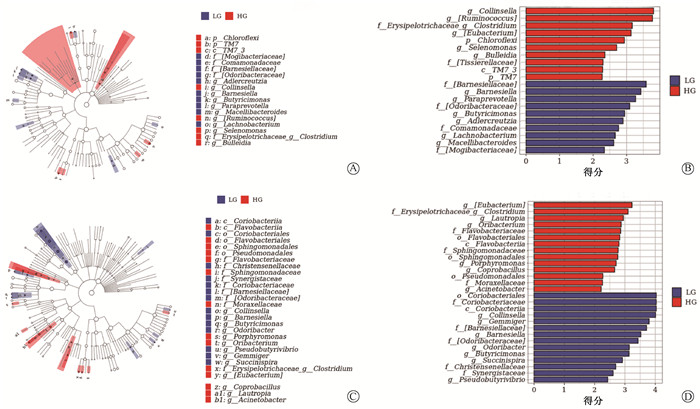
|
注:A、C为分支图,展示了从门到属的主要分类单元的分类等级关系;B、D为柱状图,可直观看出各分类单元的LDA分析对数得分值。LDA阈值为2,P<0.05 图 2 低症状级别组(LG)与高症状级别组(HG)物种差异LEfSe (LDA Effect Size)分析 A、B.放疗前样本LG组与HG组的LEfSe (LDA Effect Size)分析;C、D.放疗后样本LG组与HG组的LEfSe分析 Figure 2 LDA Effect Size (LEfSe) analysis of species differences between the LG and HG groups A, B. LEfSe analysis of pre-radiotherapy samples of the LG and HG groups; C, D. LEfSe analysis of post-radiotherapy samples of the LG and HG groups |
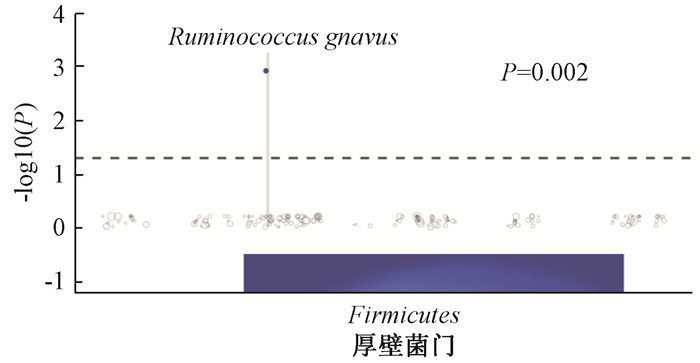
|
注:放疗前样本高症状级别组较低症状级别组上调。下方是物种所属门phylum,虚线上方实点代表上调具有显著性的物种species,纵坐标为显著性的-log10 P值),可以看到Ruminococcus gnavus显著上调(P=0.002) 图 3 低症状级别组(LG)与高症状级别组(HG)物种差异MetagenomeSeq分析 Figure 3 Metagenomeseq analysis of species differences between the LG and HG groups |
|
|
表 2 LG组和HG组放疗前较放疗后显著上调的物种MetagenomeSeq分析 Table 2 Metagenomeseq analysis of the species significantly upregulated before radiotherapy compared with that after radiotherapy in the LG and HG groups |
2、根治性放疗与术后放疗肠道微生物群的差异
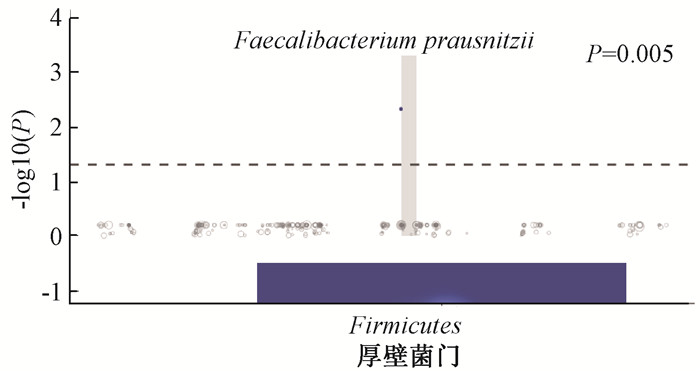
在20例RR患者中有9例HG患者,在17例PR患者中有10例HG。此外,RR和PR患者中肠道微生物群的丰度和组成存在差异。在放疗前样本中,RR的α多样性高于PR,但差异无统计学意义(Chao1:P=0.091,Shannon:P=0.051,图 4A),放疗后差异明显(Chao1:P=0.002,Shannon:P=0.027,图 4C)。两组间的β多样性通过NMDS分析也存在差异(放疗前stress=0.161,放疗后stress=0.16,图 4B、D)。在种的水平上通过MetagenomeSeq分析发现放疗前RR组样本中的Faecalibacterium prausnitzii明显高于PR组(P=0.005,图 5)。
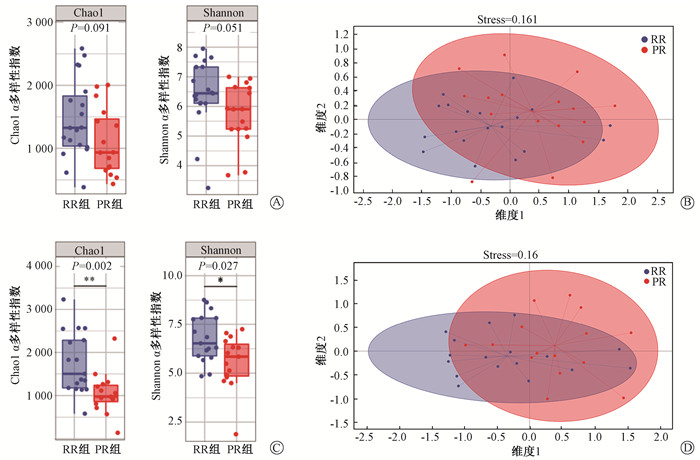
|
注:Stress<0.2说明结果可靠 图 4 根治性放疗组(RR)与术后放疗组(PR)微生物多样性分析 A、C.放疗前和放疗后RR组与PR组α多样性对比箱线图;B、D.放疗前和放疗后RR组与PR组基于Unweighted-Unifrac的NMDS分析 Figure 4 Microbial diversity analysis of the RR and PR groups A, C. Box-plots of α diversity comparison between the RR and PR groups before and after radiotherapy; B, D. NMDS analysis based on Unweighted-Unifrac between the RR and the PR groups before and after radiotherapy |
|
注:放疗前样本RR组较PR组上调。下方是物种所属门phylum,虚线上方实点代表上调具有显著性的物种species,纵坐标为显著性的-log10 P值。图中Faecalibacterium prausnitzii显著上调(P=0.005); 图 5 根治性放疗组(RR)与术后放疗组(PR)物种差异MetagenomeSeq分析 Figure 5 Metagenomeseq analysis of species differences between the RR and PR groups |
3、宫颈癌与子宫内膜癌之间的肠道微生物差异
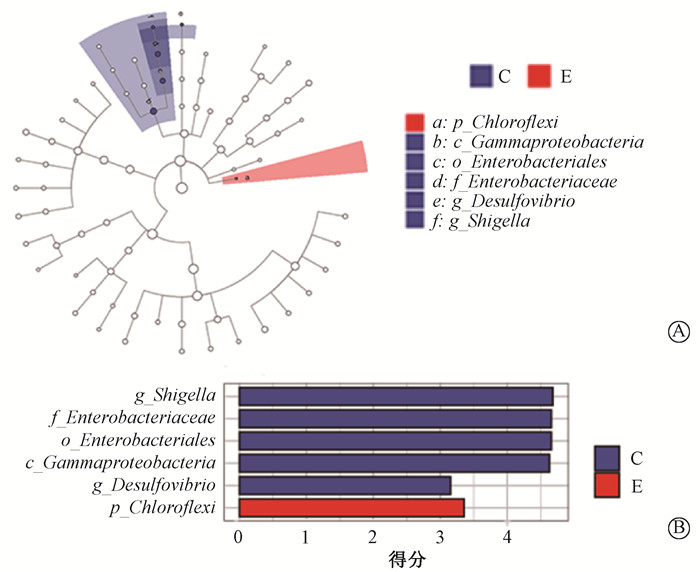
宫颈癌与子宫内膜癌患者之间的肠道微生物也存在细微差别,通过LEfSe分析发现放疗前的样本中,宫颈癌患者肠道微生物中Shigella属、Enterobacteriaceae科、Enterobacteriales目、Gammaproteobacteria纲、Desulfovibrio属等丰度相对较高;而子宫内膜癌患者肠道微生物中Chloroflexi门相对丰度更高(图 6 A、B)。
|
注:C.宫颈癌;E.子宫内膜癌 图 6 宫颈癌与子宫内膜癌放疗前样本的物种差异LEfSe (LDA Effect Size)分析A.分支图;B.柱状图 Figure 6 LDA effect size (LEfSe) analysis of species differences between cervical cancer samples and endometrial cancer samples before radiotherapy A. Cladistic diagram; B. Bar chart |
讨论
在之前的几项临床研究中,表明了盆腔放射治疗会使患者的肠道微生物发生改变,并且患者肠道微生物的组成与丰度与放射性肠损伤的发生与发展有关。2013年的一项样本量为9例妇科肿瘤患者的临床研究表明盆腔放射治疗后患者肠道微生物中Firmicutes门和Fusobacterium属分别显着降低了10%和增高了3%[13]。Wang等[14]在一项样本量为18例宫颈癌患者的研究中指出患者肠道微生物中Coprococcus属的富集与放疗后放射性肠炎的发生相关。Reis Ferreira等[15]得出Clostridium IV属、Roseburia属和Phascolarctobacterium属与前列腺癌患者放疗后放射性肠炎的关系较大的结论。Mitra等[16]在2020年的一项样本量为35例宫颈癌患者的研究中也发现对放射治疗所表现出高毒性与低毒性的患者肠道微生物组成及丰度也有着较大差异。近年来,益生菌的应用[21-24]及粪便移植的尝试[25-26]也进一步揭露了肠道菌群与放射性肠炎的关系。但是目前国内已发表的类似临床研究较少。
根据临床观察,多数发生放射性肠炎的患者在接受放疗3周左右开始出现症状。因此,本研究在患者接受15次即3周左右放射治疗时收集患者大便样本并记录患者肠道症状,以更灵敏的方式探索患者的肠道微生物特征与患者的辐射不良反应耐受能力的关系,采用了16SrRNA测序的最新技术手段,尝试通过宫颈癌及子宫内膜癌患者的肠道微生物状态对放疗期间放射性肠炎的发生及严重程度做出预测,并分析了放射性肠炎刚发生时不同严重程度的患者的肠道微生物差别,对于宫颈癌及子宫内膜癌患者放射性肠炎的微生物疗法及预防有一定的启示作用。
Manichanh等[27]及Wang等[28]分别以样本量为10例及11例腹盆部肿瘤患者的研究证实盆腔放疗引起的腹泻症状与放疗前患者肠道微生物特征存在着密切联系。这与本研究探索放射性肠炎的原则基本相同,即患者肠道初始微生物的特征与患者接受放射治疗后是否发生并发症相关。本研究发现Ruminococcus gnavus这一物种在放疗后更易发生严重放射性肠炎的患者初始肠道微生物中更高。Reis Ferreira等[15]及Mitra等[16]发现肠道微生物的多样性低与患者放射性肠炎的关系较大,尽管前者研究为前列腺癌患者,并且有研究表明性别也与放射治疗后的并发症有着一定的关系[29]。本研究也类似地发现肠道微生物多样性更低的宫颈癌与子宫内膜癌患者更易发生较为严重的放射性肠炎,并且在经过3周放射治疗后患者肠道微生物的改变也与放射性肠炎的发生及症状的轻重有关。在本研究中,症状轻、重两组在3周放疗之后,肠道微生物多样性差异变小,这可能与一些放射性肠炎较为严重的患者常规服用药物有关。2020年的一项样本量为34例宫颈癌患者的横断面研究发现,放疗之后按是否存在放射性肠损伤分组,患者有着不同特征的肠道微生物[30]。结合本研究的结果与之前的研究结论,发现Clostridiales与放射治疗的关系十分密切,前文提到的大多数物种都在其所属下。因此,放射治疗与Clostridiales的关系可进一步进行研究。
此外,本研究还发现根治性放疗组(RR)和术后放疗组(PR)在肠道微生物组成上也存在差异。放疗前PR组与RR组相比接受了子宫切除手术,大部分也接受了化疗。并且之前也有研究发现化疗等治疗方式会对患者肠道微生物产生影响[31-32]。本研究发现在放疗前样本中,RR组的α多样性高于PR组,并且在放疗3周后差异有所增加,而且两组β多样性也存在差异。另外,通过MetagenomeSeq分析发现放疗前RR组中Faecalibacterium prausnitzii这一物种显著高于PR组。而这一物种刚好也是被发现放疗前较放疗后显著上调的物种,也就是说它可能与辐射不良反应呈负相关。并且20例RR中有9例为HG,而17例PR中有10例为HG。或许Faecalibacterium prausnitzii可作为辐射抵抗性更高的患者的一种标志物种。巧合的是,Lapiere等[33]发现预防性Faecalibacterium prausnitzii的应用限制了辐射引起的细胞旁通透性过高以及结肠黏膜中嗜中性粒细胞的浸润,降低了隐窝形态学改变的严重性,即此物种对放射性肠炎有一定的保护作用。宫颈癌及子宫内膜癌患者的手术等治疗可能导致了该物种的降低,从而降低了患者的辐射耐受能力。临床上可能更加关注根治性放疗患者的肠道症状,因为此类患者通常所接受放射剂量更大。但本研究发现或许从放疗开始时,术后组患者的肠道辐射耐受性就更低,更易发生放射性肠炎。当然,这还需要进一步的研究加以证实。另外,本研究也发现宫颈癌与子宫内膜癌患者之间的肠道微生物略有不同,或许患者的不同病种也导致了患者存在不同特征的肠道微生物。尽管此差异相对较小。
本研究发现宫颈癌与子宫内膜癌患者的肠道微生物特征与放射治疗期间放射性肠炎的发生及严重程度高度相关,并且宫颈癌及子宫内膜癌接受手术等治疗后可能改变了肠道微生物组成并进一步导致了其对放射治疗耐受能力的降低。
利益冲突 无
作者贡献声明 姜海红负责实验的设计及实施、文章的撰写;李小凡、王建六负责实验的设计和指导及文章修改;尤静、古小璇协助实验临床资料的收集
| [1] |
Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients[J]. Lancet Oncol, 2007, 8(11): 1007-1017. DOI:10.1016/S1470-2045(07)70341-8 |
| [2] |
Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy-pathogenesis, treatment and prevention[J]. Nat Rev Gastroenterol Hepatol, 2014, 11(8): 470-479. DOI:10.1038/nrgastro.2014.46 |
| [3] |
Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study[J]. Gynecol Oncol, 2004, 92(3): 744-751. DOI:10.1016/j.ygyno.2003.11.048 |
| [4] |
Wang Y, Kong W, Lv N, et al. Incidence of radiation enteritis in cervical cancer patients treated with definitive radiotherapy versus adjuvant radiotherapy[J]. J Cancer Res Ther, 2018, 14. DOI:10.4103/0973-1482.163762 |
| [5] |
Jandhyala SM, Talukdar R, Subramanyam C, et al. Role of the normal gut microbiota[J]. World J Gastroenterol, 2015, 21(29): 8787-8803. DOI:10.3748/wjg.v21.i29.8787 |
| [6] |
Tuddenham S, Sears CL. The intestinal microbiome and health[J]. Curr Opin Infect Dis, 2015, 28(5): 464-470. DOI:10.1097/QCO.0000000000000196 |
| [7] |
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease[J]. N Engl J Med, 2016, 375(24): 2369-2379. DOI:10.1056/NEJMra1600266 |
| [8] |
Villéger R, Lopès A, Carrier G, et al. Intestinal microbiota: A novel target to improve anti-tumor treatment?[J]. Int J Mol Sci, 2019, 20(18): 4584. DOI:10.3390/ijms20184584 |
| [9] |
Ma W, Mao Q, Xia W, et al. Gut microbiota shapes the efficiency of cancer therapy[J]. Front Microbiol, 2019, 10: 1050. DOI:10.3389/fmicb.2019.01050 |
| [10] |
Kim YS, Kim J, Park SJ. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy[J]. Anaerobe, 2015, 33: 1-7. DOI:10.1016/j.anaerobe.2015.01.004 |
| [11] |
Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, et al. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction[J]. Gut, 2018, 67(1): 97-107. DOI:10.1136/gutjnl-2017-313789 |
| [12] |
Zhao Z, Cheng W, Qu W, et al. Antibiotic alleviates radiation-induced intestinal injury by remodeling microbiota, reducing inflammation, and inhibiting fibrosis[J]. ACS Omega, 2020, 5(6): 2967-2977. DOI:10.1021/acsomega.9b03906 |
| [13] |
Nam YD, Kim HJ, Seo JG, et al. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing[J]. PLoS One, 2013, 8(12). DOI:10.1371/journal.pone.0082659 |
| [14] |
Wang Z, Wang Q, Wang X, et al. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy[J]. J Cell Mol Med, 2019, 23(5): 3747-3756. DOI:10.1111/jcmm.14289 |
| [15] |
Reis Ferreira MR, Andreyev HJN, Mohammed K, et al. Microbiota- and radiotherapy-induced gastrointestinal side-effects (MARS) study: A large pilot study of the microbiome in acute and late-radiation enteropathy[J]. Clin Cancer Res, 2019, 25(21): 6487-6500. DOI:10.1158/1078-0432.CCR-19-0960 |
| [16] |
Mitra A, Grossman Biegert GW, Delgado AY, et al. Microbial diversity and composition is associated with patient-reported toxicity during chemoradiation therapy for cervical cancer[J]. Int J Radiat Oncol Biol Phys, 2020, 107(1): 163-171. DOI:10.1016/j.ijrobp.2019.12.040 |
| [17] |
Department of Health and Human Services, U.S. Common terminology criteria for adverse events (CTCAE) version 5.0[S]. [2017-11-27]. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
|
| [18] |
Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2:High-resolution sample inference from Illumina amplicon data[J]. Nat Methods, 2016, 13(7): 581-583. DOI:10.1038/nmeth.3869 |
| [19] |
Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation[J]. Genome Biol, 2011, 12(6): R60. DOI:10.1186/gb-2011-12-6-r60 |
| [20] |
Zgadzaj R, Garrido-Oter R, Jensen DB, et al. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities[J]. Proc Natl Acad Sci USA, 2016, 113(49): E7996-E8005. DOI:10.1073/pnas.1616564113 |
| [21] |
Linn YH, Thu KK, Win N. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study[J]. Probiotics Antimicrob Proteins, 2019, 11(2): 638-647. DOI:10.1007/s12602-018-9408-9 |
| [22] |
Okawa T, Niibe H, Arai T, et al. Effect of LC9018 combined with radiation therapy on carcinoma of the uterine cervix. A phase III, multicenter, randomized, controlled study[J]. Cancer, 1993, 72(6): 1949-1954. DOI:10.1002/1097-0142(19930915)72:6<1949::aid-cncr2820720626>3.0.co;2-w |
| [23] |
Delia P, Sansotta G, Donato V, et al. Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation[J]. Am J Gastroenterol, 2002, 97(8): 2150-2152. DOI:10.1111/j.1572-0241.2002.05946.x |
| [24] |
Chitapanarux I, Chitapanarux T, Traisathit P, et al. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients[J]. Radiat Oncol, 2010, 5: 31. DOI:10.1186/1748-717X-5-31 |
| [25] |
Cui M, Xiao H, Li Y, et al. Faecal microbiota transplantation protects against radiation-induced toxicity[J]. EMBO Mol Med, 2017, 9(4): 448-461. DOI:10.15252/emmm.201606932 |
| [26] |
Ding X, Li Q, Li P, et al. Fecal microbiota transplantation: A promising treatment for radiation enteritis?[J]. Radiother Oncol, 2020, 143: 12-18. DOI:10.1016/j.radonc.2020.01.011 |
| [27] |
Manichanh C, Varela E, Martinez C, et al. The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea[J]. Am J Gastroenterol, 2008, 103(7): 1754-1761. DOI:10.1111/j.1572-0241.2008.01868.x |
| [28] |
Wang A, Ling Z, Yang Z, et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study[J]. PLoS One, 2015, 10(5): e0126312. DOI:10.1371/journal.pone.0126312 |
| [29] |
Cui M, Xiao H, Li Y, et al. Sexual dimorphism of gut microbiota dictates therapeutics efficacy of radiation injuries[J]. Adv Sci (Weinh), 2019, 6(21): 1901048. DOI:10.1002/advs.201901048 |
| [30] |
盛翔, 朱瑞娟, 李苏宜. 肠道微生态与宫颈癌合并放射性肠损伤的临床研究[J]. 肠外与肠内营养, 2020, 27(2): 78-83. Sheng X, Zhu RJ, Li SY. Clinical study of intestinal microecology and intestinal radiation injury in cervical cancer patients[J]. Parenteral Enteral Nutrition, 2020, 27(2): 78-83. DOI:10.16151/j.1007-810x.2020.02.004 |
| [31] |
Deleemans JM, Chleilat F, Reimer RA, et al. The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult cancer survivors; study protocol[J]. BMC Cancer, 2019, 19(1): 1243. DOI:10.1186/s12885-019-6473-8 |
| [32] |
Chattopadhyay I, Nandi D, Nag A. The pint-sized powerhouse: Illuminating the mighty role of the gut microbiome in improving the outcome of anti-cancer therapy[J]. Semin Cancer Biol, 2021, 70: 98-111. DOI:10.1016/j.semcancer.2020.07.012 |
| [33] |
Lapiere A, Geiger M, Robert V, et al. Prophylactic Faecalibacterium prausnitzii treatment prevents the acute breakdown of colonic epithelial barrier in a preclinical model of pelvic radiation disease[J]. Gut Microbes, 2020, 12(1): 1-15. DOI:10.1080/19490976.2020.1812867 |
 2021, Vol. 41
2021, Vol. 41


