根据肿瘤原发部位,胃癌可分为食管胃结合部癌和非结合部癌,其中后者约占胃癌总体患者的70%[1]。2019年中国临床肿瘤学会(CSCO)胃癌诊疗指南提出,术后辅助放疗在局部进展期非食管胃结合部癌中的治疗效果尚不明确,需对不同亚组行预后分析研究以期为临床治疗提供科学证据[2]。虽然国际抗癌联盟(UICC)分期建议胃癌术中淋巴结清扫数目应>15枚[3],但临床上由于各种原因,常出现术后受检淋巴结不超15枚的情况。据研究报道,胃癌患者在受检淋巴结数目不超15枚的情况下,淋巴结转移率(metastatic lymph node ratio, MLR)对其预后有较好的预测效果[4-6],但它能否指导术后不同辅助治疗方案的选择则有待研究。本研究通过对美国SEER癌症数据库中淋巴结清扫不超15枚的Ⅲ期非食管胃结合部胃癌(以下统称“胃癌”)患者资料进行深入分析,探讨不同MLR的Ⅲ期胃癌患者接受术后辅助放疗的意义。
资料与方法1.数据来源: 本研究的患者资料来源于2010—2016年美国SEER 18数据库中接受胃癌术后辅助化疗或术后辅助放化疗的的Ⅲ期胃癌患者的临床信息。本研究纳入标准: ①原发部位为非食管胃结合部胃癌。②诊断时间为2010—2016年。③仅接受胃癌手术同时联合辅助化疗。④病理分期为Ⅲ期(第7版TNM分期)。⑤清扫淋巴结数目≤15枚。排除标准: ①食管胃结合部癌。②合并其他恶性肿瘤。③清扫淋巴结数目不详。④阳性淋巴结数目不详。⑤生存情况和治疗方式不详。这项研究是基于SEER数据库公开的临床资料,并且本研究已获准使用SEER数据库(10912~Nov2019)。
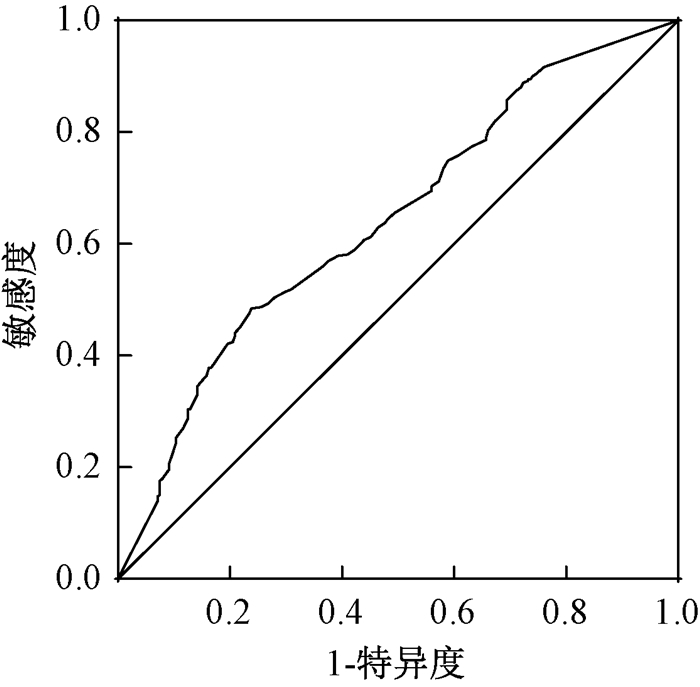
2.MLR的截点选取:MLR=阳性淋巴结数/清扫淋巴结总数。应用受试者工作特征(ROC)曲线及曲线下面积(AUC)确定MLR的最佳截点(灵敏度与特异度之和减去1的最大点即为约登指数最大点)。全组患者MLR值为0~1,以MLR值大小为检验变量,生存情况为状态变量,绘制ROC曲线,评估MLR预测预后的能力。ROC曲线分析结果显示,当MLR取值为0.527时,约登指数最大,以0.527为最佳截点值,评估全组患者预后的敏感度为0.518,特异性为0.695,AUC为0.642(95%CI: 0.597~0.687,P<0.001),见图 1。根据ROC曲线分析结果并结合既往相关报道[7-8],本研究将所有入组患者分为MLR≤0.5和>0.5两组。
|
图 1 淋巴结转移率的受试者工作特征曲线分布图 Figure 1 ROC curve distribution of MLR values |
3. 统计学处理: 根据入组患者术后是否接受辅助放疗分为手术+化疗组和手术+放化疗组,并利用R语言在手术+化疗组和手术+放化疗组之间进行1 ∶1倾向得分匹配(propensity score matching, PSM)。采用SPSS 23.0统计软件进行数据处理,组间临床因素的差异采用χ2检验,Kaplan~Meier生存分析法分析预后,Log-rank法分析组间及各亚组的总生存(overall survival, OS)差异,多因素Cox回归法评估各临床因素对预后的影响。P<0.05为差异有统计学意义。
结果1. 临床资料结果:依据入组标准共纳入590例淋巴结清扫不超15枚的Ⅲ期胃癌患者,经1 ∶1PSM后,共有488例Ⅲ期胃癌术后患者配对,其中年龄20~75岁,中位年龄55岁;男288例,女200例;腺癌328例,非腺癌160例, 其中非腺癌主要包括黏液腺癌和印戒细胞癌等病理类型;MLR≤0.5者264例和MLR>0.5者224例,见表 1。
|
|
表 1 两组Ⅲ期胃癌患者PSM前后一般临床资料比较 Table 1 Comparison of general clinical data between two groups of patients with stage Ⅲ gastric cancer before and after PSM |
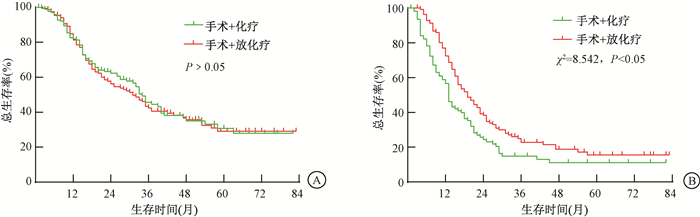
2.不同MLR的Ⅲ期胃癌患者术后放疗预后分析结果: 对本组淋巴结清扫不超15枚的Ⅲ期胃癌患者汇总分析发现,手术+放化疗组中位生存期为23个月,1、3、5年的OS率分别为77.1%、33.2%和22.8%,手术+化疗组中位生存期为21个月,1、3、5年的OS率分别为72.2%、33.6%和23.1%,两组比较差异无统计学意义(P>0.05)。亚组分析发现,MLR≤0.5患者中手术+放化疗组和手术+化疗组的OS差异无统计学意义(P>0.05),而MLR>0.5患者中手术+放化疗组的OS显著高于手术+化疗组(χ2=8.542, P<0.05),见图 2。多因素Cox回归分析显示, 种族、T分期、N分期、MLR和辅助放疗是影响淋巴结清扫不超15枚的Ⅲ期胃癌患者OS的重要因素(Wald=8.544、7.547、10.925、18.047、10.715, P<0.05),见表 2。
|
注: MLR. 淋巴结转移率 图 2 两种不同治疗方式的Ⅲ期胃癌患者生存曲线 A. MLR≤0.5; B. MLR>0.5 Figure 2 Survival curves of patients with stage Ⅲ gastric cancer with two different treatment modalities A. MLR≤0.5; B. MLR>0.5 |
|
|
表 2 PSM前后影响Ⅲ期胃癌患者预后的多因素Cox回归分析 Table 2 Multivariate Cox regression analysis of prognosis in patients with stage Ⅲ gastric cancer before and after PSM |
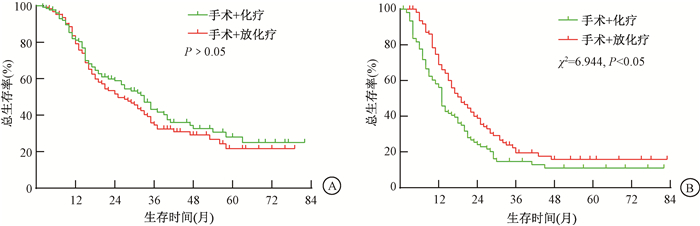
3.PSM后不同MLR的Ⅲ期胃癌患者术后放疗预后分析结果:1∶1PSM后获得244对接受手术+放化疗和手术+化疗的淋巴结清扫不超15枚的Ⅲ期胃癌患者,进一步分析发现,与PSM前的结果类似,手术+放化疗组和手术+化疗组的OS差异无统计学意义(P>0.05)。亚组分析发现,MLR≤0.5患者中手术+放化疗组和手术+化疗组的OS差异无统计学意义(P>0.05),而MLR>0.5患者中手术+放化疗组的OS显著高于手术+化疗组(χ2=6.944, P<0.05),见图 3。多因素Cox回归分析显示, 种族、T分期、N分期、MLR和辅助放疗是影响淋巴结清扫不超15枚的Ⅲ期胃癌患者OS的重要因素(Wald=7.154、8.023、7.744、17.016、4.149, P<0.05),见表 2。由此可见,本研究入组患者PSM前后的预后分析结果是相符的。
|
注: PSM. 倾向得分匹配;MLR. 淋巴结转移率 图 3 两种不同治疗方式的Ⅲ期胃癌患者PSM后生存曲线 A. MLR≤0.5; B. MLR>0.5 Figure 3 Survival curves of patients with stage Ⅲ gastric cancer with two different treatment modalities after PSM A. MLR≤0.5;B. MLR>0.5 |
3、讨论
淋巴结转移是胃癌术后患者预后不良的重要因素,目前胃癌TNM分期中的N分期主要由淋巴结转移数目来决定,但由于各级医疗机构胃癌手术方式差异导致术中淋巴结清扫数目不同,以及临床送检淋巴结数目不超都可能会造成pN分期不准确,这些情况均可造成TNM分期相同的情况下患者生存状况明显不同。据报道,在美国和中国的胃癌手术患者中,淋巴结清扫数目不超15枚的比例分别占71%和40%[9-10]。Marchet等[4]认为与清扫淋巴结>15枚相比,MLR对受检淋巴结不超15枚的预测效果更好。本研究对590例淋巴结清扫不超15枚的Ⅲ期胃癌术后患者进行了回顾性分析,多因素分析显示MLR为本组患者预后的重要影响因素,术后辅助放疗可明显改善MLR>0.5患者预后。
目前国际上对于MLR截点值的划分尚没有达成共识,不同学者选择MLR界值的方法各不相同。刘宏根等[11]是将MLR按照每5%分组,共21组,运用Log-rank检验比较各组术后生存差异,最终得出界值为≤0.1、0.1~0.3、0.3~0.6和>0.6。武优优[12]是将MLR按照四分位法分段得出界值为0~0.24、0.25~0.49、0.5~0.74和0.75~1.00。周荣健等[13]在利用MLR预测胃癌手术患者的预后时,运用了ROC曲线来评估MLR≤0.4和MLR>0.4的预测能力。本研究主要是根据ROC曲线和AUC检验结果将MLR划分为≤0.5和>0.5两组,这种划分方法具有较好的可重复性和科学性。为了更普遍地利用MLR预测胃癌手术患者的预后,未来需要大量的研究来明确MLR分级。
局部进展期胃癌术后辅助放疗目前在国内外尚存争议[14]。ARTIST-Ⅱ试验结果提示,术后辅助放疗并不能提高Ⅱ~Ⅲ胃癌患者总生存,但其亚组分析尚存在很大研究空间,亟需更多临床试验进一步确定术后辅助放疗在局部进展期胃癌治疗中的价值[15]。胃癌标准的手术方式是D2根治术[16],但我国目前50%的胃癌患者未能接受标准的D2手术[17],这会增加肿瘤的局部复发率,而术后辅助放疗可作为一种有效的手段弥补手术的不足。另外,胃癌手术患者受检淋巴结数目不足也会降低治疗效果[18],而术后辅助放疗则可能会改善患者的预后。Deng等[19]对天津医科大学肿瘤医院1 563例胃癌术后患者行回顾性分析,清扫淋巴结≤15枚的患者占39%。Wang等[20]对1 343例D2根治术胃癌患者行回顾性分析,清扫淋巴结≤15枚的患者占55.2%。在这两家国内顶级胃癌诊疗中心内,尚有将近一半患者清扫淋巴结≤15枚,那么全国范围内受检淋巴结数目≤15枚的胃癌患者数量则相当可观。因此,本研究试图探讨术后辅助放疗对淋巴结清扫不超15枚的Ⅲ期胃癌患者的预后影响,结果发现,对于总体患者而言,手术+放化疗组与手术+化疗组的OS并无明显差异,但MLR>0.5患者中手术+放化疗组的OS明显优于手术+化疗组。
本研究属于回顾性分析研究,难免存在研究设计上的局限性。本研究中胃癌手术水平、护理能力、医院级别以及患者的临床表现、营养状况等都会对结果有一定的影响,而且SEER数据库中没有登记放疗剂量及分次、化疗方案、术后并发症等可能影响预后的临床信息,但为了探讨不同MLR的Ⅲ胃癌患者选择术后辅助放疗的意义,本研究在手术+化疗组和手术+放化疗组之间进行1 ∶1 PSM,以便降低干扰因素对研究结果的影响,最终发现两组患者PSM前后的统计学结果是一致的。另外,本研究中发现SEER数据库登记的临床资料均来自初治癌症患者,因此缺乏肿瘤复发信息,未来会收集本院胃癌患者的详细资料进一步研究术后辅助放疗对局部复发的影响。
综上所述,MLR是淋巴结清扫不超15枚的Ⅲ期胃癌手术联合辅助化疗患者预后的重要影响因素,在一定程度上可以指导术后辅助放疗的选择。MLR≤0.5患者的OS并未从术后辅助放疗中获益,而MLR>0.5患者则建议接受术后辅助放疗以改善预后。
利益冲突 全体作者声明本文不存在任何利益冲突,进行该研究未接受任何不正当职务及财务获益,并对本研究的独立性和科学性予以保证
作者贡献声明 李良负责数据分析、论文撰写及校对;谢家存、王志斌负责病例收集和数据处理;梁恒坡负责数据核对和文献整理;吴广银负责选题设计和论文审核
| [1] |
胥润, 李建, 胡登敏, 等. 食管胃结合部癌与非结合部胃癌临床病理特征差异[J]. 国际外科学杂志, 2019, 46(12): 830-834. Xu R, Li J, Hu DM, et al. Clinicopathological differences between patients with adenocarcinoma of esophagogastric junction and gastric cancer without esophagogastric junction invasion[J]. Int J Surg, 2019, 46(12): 830-834. DOI:10.3760/cma.j.issn.1673-4203.2019.12.009 |
| [2] |
中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)胃癌诊疗指南2019[M]. 北京: 人民卫生出版社, 2019. Guidelines Working Committee of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO) guidelines for diagnosis and treatment of gastric cancer 2019[M]. Beijing: People's Medical Publishing House, 2019. |
| [3] |
Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging handbook: from the AJCC cancer staging manual[M]. 7th edition. NewYork: Springer, 2010.
|
| [4] |
Marchet A, Mocellin S, Ambrosi A, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in alarge, multicenter series[J]. Eur J Surg Oncol, 2008, 34(2): 159-165. DOI:10.1016/j.ejso.2007.04.018 |
| [5] |
Xiao LB, Yu JX, Wu WH, et al. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer[J]. World J Gastroenterol, 2011, 17(46): 5123-5130. DOI:10.3748/wjg.v17.i46.5123 |
| [6] |
Sun Z, Zhu GL, Lu C, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancerpatients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients[J]. Ann Oncol, 2009, 20(5): 897-905. DOI:10.1093/annonc/mdn707 |
| [7] |
Kutlu OC, Watchell M, Dissanaike S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients[J]. Surg Oncol, 2015, 24(2): 84-88. DOI:10.1016/j.suronc.2015.03.001 |
| [8] |
刘永宁. 淋巴结转移率在进展期胃癌患者中的预后价值[D]. 济南: 山东大学, 2016. Liu YN. Prognostic value of metastatic lymph nodes ratio in advanced gastric cancer patients[D]. Jinan: Shandong University, 2016. |
| [9] |
Liu H, Deng J, Zhang R, et al. The RML of lymph node metastatic was superior to the LODDS for evaluating the prognosis of gastric cancer[J]. Int J Surg, 2013, 11(5): 419-424. DOI:10.1016/j.ijsu.2013.03.009 |
| [10] |
Qiu MZ, Qiu HJ, Wang ZQ, et al. The tumor-log odds of positive lymph nodes-metastatic staging system, a promising new staging system for gastric cancer after D2 resection in China[J]. PLoS One, 2012, 7(2): e31736. DOI:10.1371/journal.pone.0031736 |
| [11] |
刘宏根, 梁寒, 邓靖宇, 等. 淋巴结转移率对淋巴结清扫不足15枚胃癌患者预后评估的价值[J]. 中华胃肠外科杂志, 2013, 16(2): 151-154. Liu HG, Liang H, Deng JY, et al. Prognostic value of metastatic lymph node ratio for gastric cancer patients with less than 15 lymph nodes dissection[J]. Chin J Gastrointest Surg, 2013, 16(2): 151-154. DOI:10.3760/cma.j.issn.1671-0274.2013.02.016 |
| [12] |
武优优. 淋巴结转移率评估T4期胃癌预后的价值研究[D]. 太原: 山西医科大学, 2015. Wu YY. The research of the value that the rate of lymph node metastatic evaluated the prognosis of patients with T4 gastric cancer[D]. Taiyuan: Shanxi Medical University, 2015. |
| [13] |
周荣健, 张恒, 束平, 等. Ⅲ期胃癌根治术后预后因素分析及淋巴结转移率对预后的预测价值(附995例报告)[J]. 中华消化外科杂志, 2019, 18(3): 250-258. Zhou RJ, Zhang H, Shu P, et al. Prognostic factors of radical gastrectomy for stage Ⅲ gastric cancer and predictive value of metastatic lymph node ratio for prognosis: a report of 995 cases[J]. Chin J Dig Surg, 2019, 18(3): 250-258. DOI:10.3760/cma.j.issn.1673-9752.2019.03.010 |
| [14] |
王鑫, 金晶. 胃癌术后辅助放疗的利与弊[J]. 中华胃肠外科杂志, 2015, 18(10): 986-989. Wang X, Jin J. Advantages and disadvantages of postoperative radiotherapy inlocally advanced gastric cancer[J]. Chin J Gastrointest Surg, 2015, 18(10): 986-989. DOI:10.3760/cma.j.issn.1671-0274.2015.10.005 |
| [15] |
Dai Q, Jiang L, Lin RJ, et al. Adjuvant chemoradiotherapy versus chemotherapy for gastric cancer: a meta-analysis of randomized controlled trials[J]. J Surg Oncol, 2015, 111(3): 277-284. DOI:10.1002/jso.23795 |
| [16] |
Ajani JA, D'Amico TA, Almhanna K, et al. Gastric cancer, Version 3.2016, NCCN clinical practice guidelines in oncology[J]. J Natl Comp Canc Netw, 2016, 14(10): 1286-1312. DOI:10.6004/jnccn.2016.0137 |
| [17] |
王贵娟, 李建彬. 胃癌辅助放疗研究现状与进展[J]. 中华肿瘤防治杂志, 2020, 27(11): 915-920. Wang GJ, Li JB. Research status and progress of adjuvant radiotherapy for gastric cancer[J]. Chin J Cancer Prev Treat, 2020, 27(11): 915-920. DOI:10.16073/j.cnki.cjcpt.2020.11.12 |
| [18] |
Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database[J]. J Clin Oncol, 2005, 23(28): 7114-7124. DOI:10.1200/JCO.2005.14.621 |
| [19] |
Deng J, Zhang R, Pan Y, et al. Comparison of the staging of regional lymph nodes using the sixth and seventh editions of the tumor-node-metastatic (TNM) classification system for the evaluation of overall survival in gastric cancer patients: findings of a case-control analysis involving a single institution in China[J]. Surgery, 2014, 156(1): 64-74. DOI:10.106/j.surg.2014.03.020 |
| [20] |
Wang W, Xu DZ, Li YF, et al. Tumor-ratio-metastatic staging system as an alternative to the 7th edition UICC TNM system in gastric cancer after D2 resection-results of a single-institution study of 1343 Chinese patients[J]. Ann Oncol, 2011, 22(9): 2049-2056. DOI:10.1093/annonc/mdq716 |
 2021, Vol. 41
2021, Vol. 41


