2. 许昌市鄢陵县中心医院, 许昌 461200;
3. 香港理工大学, 香港 999077
2. Yanling County Central Hospital, Xuchang City, Xuchang 461200, China;
3. Department of Health Technology and Informatics, Hong Kong Polytechnic University, Hong Kong SAR 999077, China
术后放疗可以提高乳腺癌术后患者的局部区域控制率,降低死亡率,是乳腺癌的重要治疗手段[1-2],而如何降低正常器官的放疗受照射剂量、减少每次治疗时间是医生和患者关心的重点。由于乳腺癌患者治疗后生存时间较长,因此对乳腺癌患者危及器官的保护也越来越受关注,包括心脏、肺以及健侧乳腺、臀丛神经等危及器官,特别是心脏的保护。有研究表明与放化疗相关心血管因素(占16.3%)是乳腺癌患者的第二大病死原因,既往有心脏病史的乳腺癌患者5年心血管病死率甚至超过肿瘤(16.9% vs. 14.6%)[3]。
乳腺癌术后放疗可采用适形放疗技术(CRT)、调强放疗(IMRT)技术、适形与调强混合照射技术、容积旋转调强(VMAT)技术等多种措施降低危及器官剂量[4-5]。VMAT技术可以提高治疗效率,在头颈部、胸腹部等很多部位肿瘤得到了广泛的应用[6-7]。对于乳腺癌,由于胸壁靶区紧邻心肺等危及器官,临床上对VMAT的应用有一定的争论,有些研究报告认为IMRT比VMAT能更好地保护肺、心脏等危及器官,但是最新的研究者多数认为VMAT在乳腺癌术放疗中的综合表现更好[8-12]。半野是非对称野的一种特殊形式,在模体内的剂量分布特性有别于对称野,在传统放疗时代应用较多,以使不同能量射野之间的剂量衔接更均匀,但在精确放疗时代,对半野的临床优势研究较少。本研究分析半野剂量分布特点,对VMAT进行分段,结合VMAT技术的优点从靶区剂量分布适形度、均匀性及两肺、心脏、心脏各亚结构、健侧乳腺、臂丛神经等受量等方面,比较乳腺癌术后放疗半野段弧VMAT与连续VMAT技术之间的剂量差异及临床实施可行性,为临床选择治疗方案提供依据。
资料与方法1. 射野离轴剂量测量:采用IBA三维水箱系统测量对称野以及半野两种射野形式的野内及野外离轴剂量分布,12 cm×16 cm射野,源皮距(SSD)为100 cm,以百分深度剂量(PDD)最大剂量点归一,采集对称野和半野两种方式射野,1.5、3、5、10、15、20、30 cm共7个深度的射野短径方向的离轴剂量。半野的设置是将一侧钨门推至射野中心并固定,形成定义为半野射野的一边,另一侧以多叶光栅(MLC)组成。对称野射野边缘定义为离轴剂量达到中心轴剂量50%位置[13],半野射野边缘定义为射野中心轴位置。
2. 病例资料:选取河南省肿瘤医院实施术后放疗的50例左侧乳腺癌患者,25例为保乳术后,25例为改良根治术后,年龄20~71岁,中位年龄49岁,保乳根治术患者病理分期pT1~2N0~1M0,均为非特殊浸润性癌Ⅱ级,改良根治术患者病理分期pT2~3N1M0,其中1例为非特殊浸润性癌伴部分髓样癌特征,其他均为非特殊浸润性癌Ⅱ级或Ⅲ级。
保乳术后病例临床靶区范围为乳腺区域,改良根治术后病例临床靶区(CTV)包括整个患侧胸壁和锁骨上区,要求处方剂量包括至少90%的(计划靶区PTV)体积,CTV在左侧、右侧、背侧3方向外扩1 cm形成PTV边界,腹侧计划评估PTV与皮肤表面一致,优化计算PTV在皮肤外扩2 cm,处方剂量为50 Gy/25次。PTV的平均体积为(805.8±230.0) cm3(289.7~1455.3 cm3)。危及器官(OAR)勾画同侧肺、心脏、左心室、左冠状动脉前降支、健侧乳腺、健侧肺、双侧臂丛神经。根据患者病情,在PTV周围的皮肤表面覆盖1 cm的补偿垫,临床关注的重要结构体积均值如PTV、患者侧、健侧肺体积分别为(884.8±199.0)、(1 082.1±265.3)、(1 307.9±249.7)cm3。
3. 体位固定及CT定位:所有患者均采用真空固定垫进行体位固定,患者双手互握置于额顶,以保证在定位及治疗时胳膊不在射束路径内。仰卧体位,自由呼吸状态,飞利浦大孔径CT(荷兰飞利浦公司)进行CT模拟定位,扫描层厚3 mm,治疗设备采用直线加速器瓦里安Truebeam(美国瓦里安公司),60对MLC,MLC叶片中间20 cm×20 cm厚度为5 mm,外周20 cm×20 cm,厚度为10 mm,铅门跟随MLC运动,6 MV X射线,每周一次治疗前采用锥形束CT进行靶区位置验证。
4. 计划设计: 对称野连续弧VMAT射野设置:295°~135°顺时针以及135°~295°逆时针两个200°弧,根据患者靶区形状光栅角在15°~35°范围调整,射束等中心点在靶区中心。半野分段弧VMAT射野设置:顺时针和逆时针两个200°弧,起始角度与连续弧VMAT一致,每个弧在机架角35°处等距拆分为两个弧,在顺时针方向,在弧第1部分,准直器铅门X2打开,X1锁定;在弧的第2部分,旋转光栅,准直器铅门X1打开,X2锁定。依据切线方向移动射野中心点包全靶区,形成半野分段弧的射野设置。在逆时针方向,射野设置思路保持不变。半野的肺侧边界由铅门和MLC共同组成,体外一侧边界则由MLC适形靶区形状。治疗计划系统为Raystation 4.6(瑞典Raysearch公司)。
5. 计划评估:比较两组计划靶区及危及器官的相关数据和治疗MU数,靶区参数为D2%、D95%、Dmean、适形指数(CI)、均匀指数(HI),用D2%表示靶区最大剂量、用D95%表示靶区最小剂量。患侧肺为V5、V10、V20、Dmean,健侧肺为V5、Dmean,健侧乳腺为Dmean,心脏为V5、V30、Dmean,以及心脏的前降支、左心室、左心房、右心室、右心房等各心脏亚结构平均剂量。适形指数CI=(TVRI)2/(TV·VRI)。其中TVRI为处方剂量覆盖的靶区体积;TV为靶区体积;VRI为处方剂量覆盖的所有体积。均匀性指数HI=(D2-D98)/D处方剂量。
6、统计学处理:采用SPSS 19.0软件,对两组计划数据的靶区和危及器官各参数以及治疗跳数(MU)均采用配对t检验分析数据。P<0.05为差异有统计学意义。
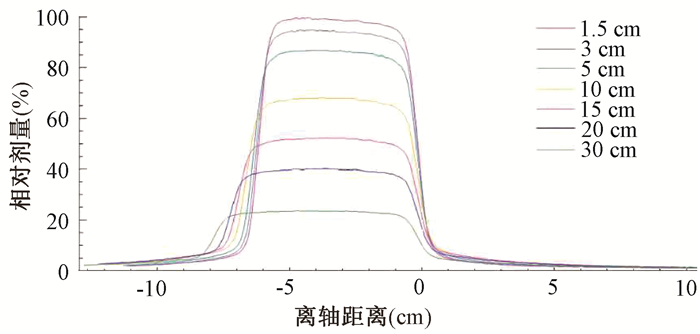
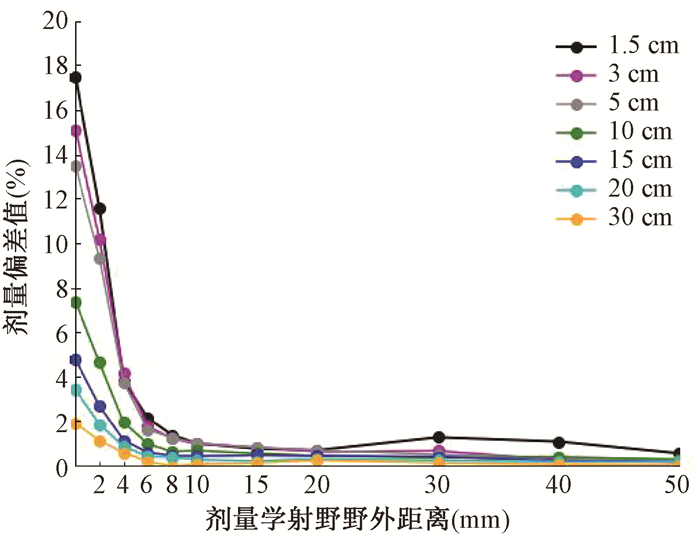
结果1.水模体离轴剂量测量结果:图 1为12 cm×16 cm射野从1.5至30 cm 7个深度的离轴剂量图,图中右侧为将铅门推至中心轴的射野边界附近不同深度的剂量结果,左侧为正常射野边界,横坐标零位处为射野中心轴,纵坐标为7个测量深度,可见随着深度的增加,对称野在模体中的射野边界因为张角因素,剂量学射野宽度在逐渐增加,至30 cm处,对称野的边界单侧从6 cm增至7.93 cm,增加将近2 cm。而将铅门设置在中轴线上形成的半野则几乎没有张角效应,射线垂直进入水模内,所有7个测量深度野外剂量线近似重叠在中轴线的射野边界处,野外剂量快速跌落。剂量学照射野的野外剂量分布对称野剂量高于半野,两者的差值见图 2,图中数值的计算方法为相同深度处对称野剂量与半野剂量差值的百分比,在距射野外缘6 mm以内有较明显的差别,愈近射野边缘差别越大。
|
图 1 水模体半野短径方向7个深度的离轴剂量分布 Figure 1 Off-axis dose distribution at 7 depth units in the short axis direction of half field |
|
图 2 水模体半野和对称野不同测量深度剂量学照射野野外剂量偏差值 Figure 2 Different measurement depths of half field and symmetric field dosimetry field dose deviation value |
2.临床剂量数据比较结果:连续弧、半野分段弧两种技术的靶区剂量体积比较和治疗MU数比较见表 1。两种计划的剂量覆盖近似一致,半野分段弧的治疗MU数均值低于连续弧技术,差异无统计学意义(P>0.05)。PTV、危及器官、左右臂丛神经、健侧乳腺比较,差异无统计学意义(P>0.05)。半野分段弧优势较大,当前临床重点关注的左肺、心脏、左冠脉前降支剂量均值都有较大幅度减少(t=-4.11、-4.42、-8.41,P=0.00)。
|
|
表 1 50例乳腺癌根治术后患者连续弧和半野分段弧计划的PTV剂量及危及器官参数配对比较(x±s) Table 1 Paired comparison of organ-at-risk parameters and PTV dose parameters for continuous arc and half-field segmented arc plans of 50 patients with breast cancer after radical operation(x±s) |
讨论
将加速器钨门固定在中轴线形成半野,射线垂直进入模体,在模体内形成特有的剂量分布,在传统放疗时代,半野技术常用于两个照射野之间的连接,使相邻的两个照射野相接于中轴线处,避免了射野之间的重叠或相离引起的热点或冷点,在连接处提供了剂量的均匀性、可靠性[14]。在精确放疗时代,也逐步有研究半野在临床放疗中的应用,如Tomida等[15]确认了半野FFF射束得到与传统物理楔形板同样的剂量梯度,可大量地减少MU数,节省照射时间,特别适用于立体定向放疗(SBRT)治疗。Lai等[16]的研究证明,相比IMRT技术,半野与VMAT的结合可以明显减少左侧乳腺癌放疗时心脏和患侧肺的受照射剂量。
适形放疗、调强放疗、容积调强放疗等精确放疗技术,均依赖于MLC的精准运动引导射线,使靶区剂量覆盖更好,危及器官剂量更低。由于MLC结构设计特点,存在小部分射线穿过MLC形成漏透射剂量,其中包括穿透过MLC非端面处叶片内透射剂量、相邻叶片间凹槽结构的漏射线,以及相对叶片合拢时端面间漏射线,叶片间漏射为1.3%~2.0%,叶片内透射率为1%左右[17-19],这部分射线必然增加危及器官的剂量。
本研究基于瓦里安Truebeam加速器进行对称野和半野的测量,发现从剂量分布和数据统计显示出两个具体方式的不同点。首先随深度增加,对称野的剂量学射野逐步扩大,30 cm模体深度处增加将近2 cm,而半野剂量学射野几乎没有增大;另外,剂量学射野的野外剂量分布,对称野也高于半野,分析原因,应该是少了MLC的穿射漏射,以及受半影以及射束张角影响,使射线在模体内的散射状态半野不同于对称野。
技术改进的目的是为了临床治疗效果的改善,与CRT和IMRT技术相比较,VMAT在乳腺癌放疗应用中有许多优势,如较高的靶区适形度和均匀性,避免了靶区内过多的剂量冷点和热点[20],较少的治疗时间减少患者因长时间保持体位固定状态引起的不适以及分次内的位置变化影响,而且治疗时间仅2~3 min,非常有利于在呼吸动度较大的部位保证患者的剂量准度。本研究利用半野的剂量学特性,配合准直器角度变化,将治疗弧分成两部分,发挥VMAT技术调制能力强的优势,使以钨门形成的半野总是处于人体方向,保护心脏及患侧肺;而以多叶光栅形成的开放野处于身体外侧,发挥基于MLC的强度调节功能,以保证靶区的剂量适形度和均匀度。
危及器官的保护是放疗技术研究的重要出发点,放射性肺炎的发生主要由肺的V5、V20、平均剂量等指标决定[21-22]。放射性心脏损伤发生关键影响因素总剂量体积,虽然目前尚无引起放射性心脏损伤确切的最小照射剂量,但较高剂量的照射显然会增加心血管事件的发生率[23-24]。Darby等[25]认为心脏平均受照剂量每增加1 Gy,发生冠状动脉疾病事件的概率就相应增加7.4%。本研究两种技术危及器官剂量体积比较, 对于患侧肺的参数为V5、V10、V20、Dmean,心脏整体结构的V5、V30、Dmean和左冠状动脉前降支、左心房、左心室等心脏亚结构剂量均值,结果表明半野分段弧技术明显优于连续弧技术。
健侧肺和健侧乳腺的剂量体积数据两种技术差异无统计学意义,数据分析结果有利于半野分段弧技术,具体数值依照Di Betta等[26]建议采用平均剂量5 Gy作为对靶区周围健康组织的优化辐射防护剂量。
两个计划都有良好的剂量适形度、剂量分布均匀度。在治疗效果方面,治疗MU数和半野分段MU均值少于连续弧,而在临床治疗执行效率方面,出束时间,平均每个患者连续弧比分段弧少约30 s,其原因为相比连续弧,分段弧在治疗时多了一个环节以进行光栅角和多叶光栅位置的重置。
综上所述,研究结果表明,与连续弧VMAT计划相比,左侧乳腺癌术后放疗采用半野分段弧VMAT计划设计对心脏及其各亚结构、左右肺、健侧乳腺等危及器官的保护更优,对保证乳腺癌患者高质量的治疗后生存更有利,具有较好的临床应用意义。
利益冲突 无
作者贡献声明 李定杰负责数据分析和论文撰写;丁丹红、魏胜涛、陈文远、黎田负责数据整理和计划设计;蔡璟负责论文修改;葛红负责研究设计及提供学术建议
| [1] |
Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer[J]. Radiother Oncol, 2015, 114(1): 3-10. DOI:10.1016/j.radonc.2014.11.030 |
| [2] |
McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials[J]. Lancet, 2014, 383(9935): 2127-2135. DOI:10.1016/S0140-6736(14)60488-8 |
| [3] |
Abdel-Qadir H, Austin PC, Lee DS, et al. A population-based study of cardiovascular mortality following early-stage breast cancer[J]. JAMA Cardiol, 2017, 2(1): 88-93. DOI:10.1001/jamacardio.2016.3841 |
| [4] |
Beckham WA, Popescu CC, Patenaude VV, et al. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer?[J]. Int J Radiat Oncol Biol Phys, 2007, 69(3): 918-924. DOI:10.1016/j.ijrobp.2007.06.060 |
| [5] |
Liu J, Ng D, Lee J, et al. Chest wall desmoid tumours treated with definitive radiotherapy: a plan comparison of 3D conformal radiotherapy, intensity-modulated radiotherapy and volumetric-modulated arc radiotherapy[J]. Radiat Oncol, 2016, 11: 34. DOI:10.1186/s13014-016-0611-0 |
| [6] |
Palma D, Vollans E, James K, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy[J]. Int J Radiat Oncol Biol Phys, 2008, 72(4): 996-1001. DOI:10.1016/j.ijrobp.2008.02.047 |
| [7] |
Verbakel WF, Cuijpers JP, Hoffmans D, et al. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study[J]. Int J Radiat Oncol Biol Phys, 2009, 74(1): 252-259. DOI:10.1016/j.ijrobp.2008.12.033 |
| [8] |
Zhao H, He M, Cheng G, et al. A comparative dosimetric study of left sided breast cancer after breast-conserving surgery treated with VMAT and IMRT[J]. Radiat Oncol, 2015, 10: 231. DOI:10.1186/s13014-015-0531-4 |
| [9] |
Jin GH, Chen LX, Deng XW, et al. A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: conventional tangential field, filed-in-filed, tangential-IMRT, multi-beam IMRT and VMAT[J]. Radiat Oncol, 2013, 8: 89. DOI:10.1186/1748-717X-8-89 |
| [10] |
Osman SO, Hol S, Poortmans PM, et al. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation[J]. Radiother Oncol, 2014, 112(1): 17-22. DOI:10.1016/j.radonc.2014.04.004 |
| [11] |
郭晨雷, 徐英杰, 戴建荣. 乳腺癌容积调强弧形治疗的射野边界外放方法[J]. 中华放射肿瘤学杂志, 2018, 27(9): 845-849. Guo CL, Xu YJ, Dai JR. Margin expansion for treatment field of volumetric modulated arc therapy for breast cancer[J]. Chin J Radiat Oncol, 2018, 27(9): 845-849. DOI:10.3760/cma.j.issn.1004-4221.2018.09.012 |
| [12] |
Hu J, Han G, Lei Y, et al. Dosimetric comparison of three radiotherapy techniques in irradiation of left-sided breast cancer patients after radical mastectomy[J]. Biomed Res Int, 2020, 2020: 7131590. DOI:10.1155/2020/7131590 |
| [13] |
Klein EE, Hanley J, Bayouth J, et al. Task Group 142 report: quality assurance of medical accelerators[J]. Med Phys, 2009, 36(9): 4197-4212. DOI:10.1118/1.3190392 |
| [14] |
Duan J, Shen S, Spencer SA, et al. A dynamic supraclavicular field-matching technique for head-and-neck cancer patients treated with IMRT[J]. Int J Radiat Oncol Biol Phys, 2004, 60(3): 959-972. DOI:10.1016/j.ijrobp.2004.06.213 |
| [15] |
Tomida T, Konno M, Urikura A, et al. Wedged field using the half-field method with a flattening filter-free photon beam[J]. Radiol Phys Technol, 2020, 13(2): 201-209. DOI:10.1007/s12194-020-00561-8 |
| [16] |
Lai Y, Chen Y, Wu S, et al. Modified volumetric modulated arc therapy in left sided breast cancer after radical mastectomy with flattening filter free versus flattened beams[J]. Medicine (Baltimore), 2016, 95(14): e3295. DOI:10.1097/MD.0000000000003295 |
| [17] |
Lorenz F, Nalichowski A, Rosca F, et al. Spatial dependence of MLC transmission in IMRT delivery[J]. Phys Med Biol, 2007, 52(19): 5985-5999. DOI:10.1088/0031-9155/52/19/018 |
| [18] |
Li J, Zhang XZ, Gui LG, et al. Clinical feasibility of leakage and transmission radiation dosimetry using multileaf collimator of ELEKTA synergy-S accelerator during conventional radiotherapy[J]. J Med Imag Health Inform, 2016, 6(2): 409-415. DOI:10.1166/jmihi.2016.1706 |
| [19] |
Kragl G, Wetterstedt S, Knäusl B, et al. Dosimetric characteristics of 6 and 10 MV unflattened photon beams[J]. Radiother Oncol, 2009, 93(1): 141-146. DOI:10.1016/j.radonc.2009.06.008 |
| [20] |
Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes[J]. Int J Radiat Oncol Biol Phys, 2010, 76(1): 287-295. DOI:10.1016/j.ijrobp.2009.05.038 |
| [21] |
Kimura T, Togami T, Takashima H, et al. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema[J]. Br J Radiol, 2012, 85(1010): 135-141. DOI:10.1259/bjr/32629867 |
| [22] |
Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT)[J]. Int J Radiat Oncol Biol Phys, 2006, 66(5): 1399-1407. DOI:10.1016/j.ijrobp.2006.07.1337 |
| [23] |
Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the childhood cancer survivor study cohort[J]. BMJ, 2009, 339: b4606. DOI:10.1136/bmj.b4606 |
| [24] |
Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer[J]. J Clin Oncol, 2010, 28(8): 1308-1315. DOI:10.1200/JCO.2008.20.2267 |
| [25] |
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer[J]. N Engl J Med, 2013, 368(11): 987-998. DOI:10.1056/NEJMoa1209825 |
| [26] |
Di Betta E, Fariselli L, Bergantin A, et al. Evaluation of the peripheral dose in stereotactic radiotherapy and radiosurgery treatments[J]. Med Phys, 2010, 37(7): 3587-3594. DOI:10.1118/1.3447724 |
 2021, Vol. 41
2021, Vol. 41


