宫颈癌术后复发严重影响患者生存[1],同步放化疗是术后有手术切缘阳性,淋巴结阳性和宫旁浸润等高危复发因素患者的标准治疗措施[2]。肿瘤体积大(large primary tumors,LPT)、淋巴血管间隙侵犯(lymphovascular space invasion,LVSI)和深间质浸润(deep stromal invasion,DSI)等中危因素[3]的患者建议行术后单纯放疗,同步化疗的疗效仍有争议[4-5]。其他如病理类型[6]、分化程度[7]和子宫内膜浸润[8]等与复发有关,这些患者是否需行辅助放疗尚无定论。调强放疗(IMRT)具有辐射剂量学、肿瘤局部控制高和较低不良反应的优势[9-10],正被广泛应用。复发风险因素、同步放化疗、辅助化疗的价值及正常组织的保护等是宫颈癌术后治疗的研究热点。本研究通过对362例有中危复发因素宫颈癌术后患者长期随访观察,回顾性分析生存情况和治疗不良反应,评估影响预后的因素。
资料与方法 1、临床资料收集2009年1月—2019年12月常州市第二人民医院收治的分期为ⅠB1~ⅡA(国际妇产科联盟2009版)宫颈癌术后患者362例。入选标准:①ⅠB1~ⅡA行宫颈根治性手术患者,包括盆腔和/或主动脉旁淋巴结清扫术,术后病理确诊为鳞癌(角化型和非角化型)、腺癌和腺鳞癌。②影像学检查未见远处转移。③有深间质浸润(DSI)、淋巴血管间隙侵犯(LVSI)和肿瘤体积大(LPT)中一项或多项危险因素。④血常规、肝肾功能等检查和脏器功能基本正常,功能状态(PS)评分≤2分。排除标准:①手术切缘阳性,有淋巴结转移和宫旁浸润者。②有放疗禁忌或既往有放疗史的患者。③伴有其他恶性肿瘤或有恶性肿瘤病史的患者。临床资料见表 1。
|
|
表 1 362例宫颈癌术后患者一般资料 Table 1 Characteristics of 362 patients with postoperative cervical carcinoma |
2、治疗方案
放疗采用调强放疗技术。①放疗靶区[11]:阴道残端上3~4 cm宫旁组织和淋巴结引流区包括髂总、髂外、髂内、闭孔、骶前区域。处方剂量:45~50 Gy/25次,1.8~2 Gy/次,常规分割。放疗于术后(61±23)d开始。②化疗方案:辅助化疗为以铂类为基础的两药方案,其中紫杉醇+铂类197例,多西紫杉醇+铂类71例,氟尿嘧啶+铂类26例,吉西他滨+铂类30例。化疗1~6程。同期化疗:单药周剂量29例,顺铂18例、奈达铂11例;两药联合化疗132例,其中同期1程119例、2程13例,方案与辅助化疗相同。
3、评价标准和随访不良反应评价,急性放疗不良反应按照美国肿瘤放射治疗协作组急性放射损伤分级标准评定;化疗不良反应根据美国癌症研究所常用药物毒性标准(V3.0版)评定。以无复发生存期(RFS)和总生存期(OS)为评价疗效指标。治疗期间每周复查血常规和肝肾功能检测。治疗结束后2年内3个月随访1次,2年后6个月随访1次。肿瘤复发被定义为通过活检或细针抽吸细胞学在组织学上存在肿瘤细胞和/或在CT扫描上短径>1 cm的淋巴结,或肿块在后续CT扫描上大小增加25%。盆腔内的复发为局部复发,包括阴道残端、膀胱、直肠、盆壁和盆腔淋巴结。盆腔外的复发为远处转移。随访截止时间为2020年12月31日。
4、统计学处理采用SPSS 24.0统计软件包进行统计学分析。数据比较采用χ2检验,复发风险采用二元logistic回归分析,多因素生存分析采用Cox回归模型,单因素生存分析采用Kaplan-Meier法和Log-rank检验。P<0.05为差异有统计学意义。
结果 1、生存与预后因素分析中位随访时间69个月,随访期间共43例死亡,其中不明原因或内科疾病死亡4例。全组患者3年和5年的OS率94.20%和88.39%,复发者的OS率为59.57%、19.14%,低于无复发者99.36%,98.73%(χ2=119.815~256.207,P<0.01)。未行辅助放疗者3年和5年的OS率分别为90.0%和82.86%,单纯放疗者为92.36%和87.78%,同步放化疗者为96.89%和91.30%,差异无统计学意义(P>0.05)。复发患者的中位生存期为35个月(95%CI:10.2~58.9个月)。多因素Cox分析发现有肿瘤≥4 cm、组织学分级低和未辅助放疗影响患者无复发生存期;肿瘤大小、分化程度和同步放化疗是影响患者总生存期的独立因素,见表 2。
|
|
表 2 362例宫颈癌术后患者总生存期和无复发生存期的Cox多因素预后分析 Table 2 Multivariate analysis of OS and RFS of 362 patients with postoperative cervical carcinoma susing a Cox model |
2、复发与危险因素分析
47例患者出现局部复发和/或远处转移,局部复发者9例,远处转移者26例,局部复发伴远处转移12例。21例局部复发部位出现在盆腔淋巴结复发8例、腹股沟淋巴结转移2例、盆腔淋巴结合并腹股沟淋巴结转移2例、盆壁复发6例和阴道残端复发3例。肺转移6例,肝转移7例,骨转移5例,腹膜后淋巴结转移12例,纵隔和/或锁骨上淋巴结转移8例,其中11例患者有多部位转移。70.21%的复发出现在术后2年内,中位复发时间17个月(95%CI为7.36~ 35.28个月)。多因素分析显示肿瘤大小和分化程度影响患者的RFS(表 2)。logistic回归分析发现肿瘤≥4 cm患者的复发风险为肿瘤 < 2 cm的3.287倍(95% CI:1.366~7.905,P=0.008)、病理分化程度低患者复发风险是高分化者的2.870倍(95% CI:1.105~7.457,P=0.03)。有深间质浸润(DSI)、淋巴血管间隙侵犯(LVSI)和病理类型为腺癌或腺鳞癌复发风险因素高于无DSI、LVSI和鳞癌,差异均无统计学意义(P>0.05)。
3、辅助放疗在术后治疗中价值分析较未行辅助放疗患者,单纯放疗减少36.7%的复发风险(95% CI:0.211~0.986,P=0.030);同步放化疗降低43.1%的复发风险(95% CI:0.256~0.972,P=0.042),并延长患者RFS和OS(表 2)。同步放化疗较未辅助放疗和单纯放疗减少了肿瘤≥4 cm和病理类型为腺癌或腺鳞癌以及低分化癌患者的复发率;单纯放疗减少了肿瘤≥4 cm者的复发率,未能降低腺癌或腺鳞癌以及低分化癌者的复发率(表 3)。不同年龄段、病理类型为鳞癌、中、高分化程度、肿瘤<4cm以及仅有深间质浸润、淋巴血管间隙侵犯之一危险因素患者行同步放化疗或单纯放疗与未辅助放疗比较,未能降低肿瘤复发率(P>0.05)。亚组分析显示在肿瘤≥4 cm患者中,单纯放疗较未辅助放疗延长了患者的OS(χ2=23.759,P=0.001),同步放化疗优于单纯放疗(χ2=7.614,P=0.006,图 1A)。在低分化癌患者中,行同步放化疗患者较单纯放化疗和未辅助放疗患者有更长的OS(χ2=5.964,P=0.049,图 1B),行单纯放疗未提高OS(P>0.05)。腺癌或腺鳞癌者行单纯放疗或同步放化疗较未行辅助放疗都未增加患者的OS(P>0.05)。与未行辅助放疗比较,同步放化疗或单纯放疗均延长了有LVSI和DSI的两项危险因素者的OS(χ2=6.055,5.997,P < 0.05,图 1C),同步放化疗和单纯放疗之间的OS差异无统计学意义(P>0.05)。LVSI或DSI单一危险因素患者术后辅助放疗未延长患者的OS(P>0.05)。
|
|
表 3 362例宫颈癌患者3种治疗方法各中危复发因素的复发率 Table 3 Recurrence rates of 362 postoperative cervical carcinoma patients with intermediate-risk factors receiving three treatment modes |
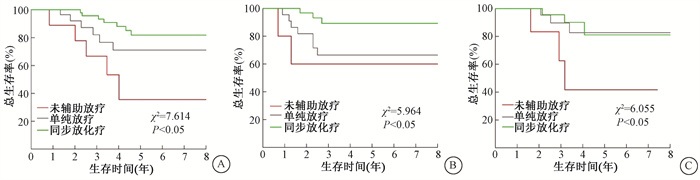
|
图 1 宫颈癌患者术后不同危险因素3种治疗模式的生存曲线A. 肿瘤≥4 cm;B.低分化癌;C有深间质浸润和淋巴血管间隙侵犯 Figure 1 Overall survival(OS)curves of the patients with different risk recurrence factors after receiving three treatment modes A. Tumor size≥4 cm; B. Poorly differentiated carcinoma; C. DSI and LVSI |
4、治疗不良反应
所有患者未出现治疗相关死亡。辅助治疗期间不良反应主要为急性期消化道和血液学不良反应,其中3级及以上的血液学不良反应、放射性肠炎和放射性膀胱炎发生率分别为9.93%、6.85%和5.82%。同步放化疗、单纯放疗和未辅助放疗患者中出现≥3级血液学不良反应人次的发生率分别为12.01%、9.20%和7.69%,3种治疗模式之间的差异均无统计学意义(P>0.05)。同步放化疗和单纯放疗的≥3级放射性肠炎发生率8.07%、5.34%,放射性膀胱炎为7.45%和3.82%,同步放化疗和单纯放疗差异无统计学意义(P>0.05)。
讨论宫颈癌患者的临床、手术情况和病理等特征决定术后复发的风险,是否需要行辅助治疗以及采用何种辅助治疗措施。目前对无高危复发风险者主要依据Sedlis标准[3]规定3项中危因素,但仍存争议。Cibula等[12]的研究显示肿瘤≥4 cm是影响患者生存期的唯一因素。因肿瘤≥4 cm的Ⅰ B期患者预后较差,国际妇产科联盟(FIGO)2018年将该类患者重新划分为Ⅰ B3期[13]。Rutledge等[14]认为ⅠB期患者预后与肿瘤大小无关,仅受LVSI和DSI影响。Cao等[15]的研究显示LVSI是影响患者生存期的唯一因素。此外,病理类型为非鳞癌[6]、病理分级低[7]的患者复发风险高。本研究发现肿瘤≥4 cm、病理低分级的患者预后较差,它们是影响患者无复发生存期和总生存期的独立因素,与近期研究相似[12, 16]。在本研究中病理类型为腺癌或腺鳞癌的患者复发风险高于鳞癌者,差异无统计学意义,可能与入组病例较少有关。
辅助放疗是减少宫颈癌患者术后复发的重要方法,对DSI、LPT和LVSI中有两项及以上因素者行辅助放疗可降低13%~46%的复发风险[3]。对有DSI单一危险因素者,Li等[17]发现辅助放疗降低患者的复发风险。肿瘤≥4 cm的患者行辅助放疗也能减少肿瘤相关死亡[12]。而Nakamura等[18]认为有中危因素的ⅠB1期者行辅助放疗无获益。本研究发现术后放疗降低了36.7%的复发风险,当患者有LVSI和DSI两项因素时,辅助放疗可减少复发率,延长患者的OS。辅助放疗减少非鳞癌者的复发率,未能改善患者RFS和OS,可能与入组病例较少有关。
同步放化疗是否使中危因素患者有进一步获益是现在争论的焦点。Ryu等[19]发现同步放化疗较单纯放疗降低2.2%复发率,提高7%无复发生存。Kim等[20]的研究显示中危因素的ⅠB~ⅡA期患者同步放化疗不比单纯放疗更有效。Li等[4]和Qin等[5]进行Meta分析也得出了不同的结论,前者认为同步放化疗能显著改善RFS和OS,而后者的分析显示同步放化疗或单纯放疗患者OS无差异。目前针对有中危因素患者术后同步放化疗的GOG 0263临床试验正在研究中,其主要目标是同步放化疗与单纯放疗相比是否显著改善生存;并分析RFS、OS和生物标志物、术后病理特征之间的关联[21],期待研究结果的披露。本研究中同步放化疗较单纯放疗进一步降低6.3%的复发风险,延长肿瘤≥4 cm和低分化患者的OS。
辅助治疗的不良反应是限制术后辅助放疗和同步放化疗运用的重要原因[18, 22],本研究中同步放化疗患者治疗过程中≥3级血液系统不良反应与单纯放疗的发生率差异无统计学意义,并经相应治疗后都能较快恢复。同步放化疗未增加3级及以上放射性肠炎和放射性膀胱炎的发生率。
总之,本研究显示肿瘤体积大、病理分化程度低是导致术后复发重要危险因素。辅助放疗改善早期宫颈癌术后有LVSI和DSI的两项危险因素患者的预后。对比单纯放疗,同步放化疗延长肿瘤体积大或低分化癌术后患者的无复发生存期和总生存期。因此认为对宫颈癌术后有复发风险的患者采用同步放化疗的治疗模式可降低复发,延长生存期,治疗不良反应可耐受。
利益冲突 所有作者声明不存在利益冲突
致谢 本研究接受常州市卫生计生委青年人才科技项目(QN201820)资助
作者贡献声明 聂斌负责数据的整理和分析、论文起草和修改;于静萍、孙威、景飞、陈光宗、李栋庆、胡莉钧、孙志强和李毅提供病例和随访;倪新初提供研究思路和研究方案
| [1] |
Sagi-Dain L, Abol-Fol S, Lavie O, et al. Cervical cancer with intermediate risk factors: is there a role for adjuvant radiotherapy? A systematic review and a Meta-Analysis[J]. Gynecol Obstet Invest, 2019, 84(6): 606-615. DOI:10.1159/000501683 |
| [2] |
Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix[J]. J Clin Oncol, 2000, 18(8): 1606-1613. DOI:10.1200/JCO.2000.18.8.1606 |
| [3] |
Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2019, 17(1): 64-84. DOI:10.6004/jnccn.2019.0001 |
| [4] |
Li M, Hu M, Wang Y, et al. Adjuvant chemoradiotherapy versus radiotherapy in cervical cancer patients with intermediate-risk factors: a systematic review and meta-analysis[J]. Eur J Obstet Gynecol Reprod Biol, 2019, 238: 1-6. DOI:10.1016/j.ejogrb.2019.04.039 |
| [5] |
Qin AQ, Liang ZG, Ye JX, et al. Significant efficacy of additional concurrent chemotherapy with radiotherapy for postoperative cervical cancer with risk factors: a systematic review and meta-analysis[J]. Asian Pac J Cancer Prev, 2016, 17(8): 3945-3951. DOI:10.14456/apjcp.2016.196/APJCP.2016.17.8.3945 |
| [6] |
Noh JM, Park W, Kim YS, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: a multicenter retrospective study (KROG 13-10)[J]. Gynecol Oncol, 2014, 132(3): 618-623. DOI:10.1016/j.ygyno.2014.01.043 |
| [7] |
Gong L, Zhang JW, Yin RT, et al. Safety and efficacy of neoadjuvant chemotherapy followed by radical surgery versus radical surgery alone in locally advanced cervical cancer patients[J]. Int J Gynecol Cancer, 2016, 26(4): 722-728. DOI:10.1097/IGC.0000000000000658 |
| [8] |
Gülseren V, Kocaer M, Çakır İ, et al. Postoperative nomogram for the prediction of disease-free survival in lymph node-negative stage Ⅰ-ⅡA cervical cancer patients treated with radical hysterectomy[J]. J Obstet Gynaecol, 2020, 40(5): 699-704. DOI:10.1080/01443615.2019.1652888 |
| [9] |
Yamamoto T, Umezawa R, Tokunaga H, et al. Clinical experience of pelvic radiotherapy or chemoradiotherapy for postoperative uterine cervical cancer using intensity-modulated radiation therapy[J]. J Radiat Res, 2020, 61(3): 470-478. DOI:10.1093/jrr/rraa004 |
| [10] |
郭旗, 许碧纯, 刘叶红, 等. 宫颈癌术后调强放射治疗的早期不良反应及影响因素[J]. 中华放射医学与防护杂志, 2020, 40(5): 365-371. Guo Q, Xu BC, Liu YH, et al. Early side effects and influencing factors of postoperative intensity-modulated radiation therapy for cervical cancer[J]. Chin J Radiol Med Prot, 2020, 40(5): 365-371. DOI:10.3760/cma.j.issn.0254-5098.2020.05.007 |
| [11] |
Toita T, Ohno T, KaneyasuY, et al. A consensus-based guideline defining the clinical target volume for pelvic lymph nodes in external beam radiotherapy for uterine cervical cancer[J]. Jpn J Clin Oncol, 2010, 40(5): 456-463. DOI:10.1093/jjco/hyp191 |
| [12] |
Cibula D, Abu-Rustum NR, Fischerova D, et al. Surgical treatment of "intermediate risk" lymph node negative cervical cancer patients without adjuvant radiotherapy-A retrospective cohort study and review of the literature[J]. Gynecol Oncol, 2018, 151(3): 438-443. DOI:10.1016/j.ygyno.2018.10.018 |
| [13] |
Lee SI, Atri M. 2018 FIGO staging system for uterine cervical cancer: enter cross-sectional imaging[J]. Radiology, 2019, 292(1): 15-24. DOI:10.1148/radiol.2019190088 |
| [14] |
Rutledge TL, Kamelle SA, Tillmanns TD, et al. A comparison of stages ⅠB1 and ⅠB2 cervical cancers treated with radical hysterectomy. Is size the real difference?[J]. Gynecol Oncol, 2004, 95(1): 70-76. DOI:10.1016/j.ygyno.2004.07.027 |
| [15] |
Cao L, Wen H, Feng Z, et al. Role of adjuvant therapy after radical hysterectomy in intermediate-risk, early-stage cervical cancer[J]. Int J Gynecol Cancer, 2021, 31(1): 52-58. DOI:10.1136/ijgc-2020-001974 |
| [16] |
Zeng J, Qu P, Hu Y, et al. Clinicopathological risk factors in the light of the revised 2018 International Federation of Gynecology and Obstetrics staging system for early cervical cancer with staging IB: a single center retrospective study[J]. Medicine (Baltimore), 2020, 99(16): e19714. DOI:10.1097/MD.0000000000019714 |
| [17] |
Li L, Song X, Liu R, et al. Chemotherapy versus radiotherapy for FIGO stages ⅠB1 and IⅡA1 cervical carcinoma patients with postoperative isolated deep stromal invasion: a retrospective study[J]. BMC Cancer, 2016, 16: 403. DOI:10.1186/s12885-016-2447-2 |
| [18] |
Nakamura K, Kitahara Y, Satoh T, et al. Analysis of the effect of adjuvant radiotherapy on outcomes and complications after radical hysterectomy in FIGO stage IB1 cervical cancer patients with intermediate risk factors (GOTIC Study)[J]. World J Surg Oncol, 2016, 14(1): 173. DOI:10.1186/s12957-016-0931-4 |
| [19] |
Ryu SY, Park SI, Nam BH, et al. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors?[J]. Int J Radiat Oncol Biol Phys, 2011, 79(3): 794-799. DOI:10.1016/j.ijrobp.2009.11.019 |
| [20] |
Kim H, Park W, Kim YS, et al. Chemoradiotherapy is not superior to radiotherapy alone after radical surgery for cervical cancer patients with intermediate-risk factor[J]. J Gynecol Oncol, 2020, 31(3): e35. DOI:10.3802/jgo.2020.31.e35 |
| [21] |
Sy R. Radiation therapy with or without chemotherapy in patients with stage I or stage Ⅱ cervical cancer who previously underwent surgery (NCT01101451)[EB/OL]. [2021-8-27]. https://clinicaltrials.gov/ct2/show/NCT01101451?cond=NCT01101451&draw=2&rank=1..
|
| [22] |
Tsuchida K, Murakami N, Kato T, et al. Postoperative pelvic intensity-modulated radiation therapy reduced the incidence of late gastrointestinal complications for uterine cervical cancer patients[J]. J Radiat Res, 2019, 60(5): 650-657. DOI:10.1093/jrr/rrz041 |
 2021, Vol. 41
2021, Vol. 41


