2. 卡罗林斯卡大学医院 卡罗林斯卡学院, 斯德哥尔摩 17177, 瑞典
2. Department of Molecular Medicine and Surgery, Karolinska Institutet, Karolinska University Hospital, Stockholm 17177, Sweden
宫颈癌的发病率在女性恶性肿瘤中位居前列,是全球女性肿瘤相关性死亡的主要原因之一[1]。手术、同步放化疗或放疗作为宫颈癌治疗的主要手段,在临床上应用广泛,然而部分患者即使接受相应治疗,仍然出现复发和死亡。因此,筛查宫颈癌患者中不良生存预后及放疗疗效差的高危人群,采取相应的分层干预治疗至关重要。
大多数实体肿瘤在有氧条件下依然依赖糖酵解,这种现象称为Warburg效应[2]。近年来多项研究表明,Warburg效应通路及其相关分子与肿瘤的预后及放射敏感性密切相关,丙酮酸脱氢酶激酶1(PDK1)、丙酮酸脱氢酶(PDH)和丙酮酸激酶M2型(PKM2)作为Warburg效应通路的关键分子在多种肿瘤组织中异常表达,在肿瘤细胞的生长增殖中发挥着重要作用,与患者预后密切相关[3-5],但在宫颈癌中的研究较少且多限于细胞水平研究。本研究通过比较PDK1、磷酸化的丙酮酸脱氢酶(p-PDH)和PKM2单独以及三分子联合高表达(PDK1high、p-PDHhigh、PKM2high及PDK1high/p-PDHhigh/PKM2high)对宫颈癌预后及术后放疗疗效的影响,以期发现最有预测价值的分子分型,为宫颈癌的个体化治疗提供新思路。
资料与方法1.患者及芯片构建:收集2003年至2012年在武汉大学中南医院接受经腹宫颈癌根治术、符合入组标准的102例患者病例资料(表 1)。所有患者未接受新辅助放疗,手术切除的原发灶组织标本用于构建组织芯片。采用2009年国际妇产科联盟(FIGO)分期,ⅠA/B期48例(ⅠA、ⅠB1和ⅠB2期分别为4、24和20例),ⅡA期38例(ⅡA1、ⅡA2期分别为13和25例),ⅡB期16例。盆腔淋巴结转移30例,其中2例伴腹主旁淋巴结转移,均通过术后病理诊断或影像学诊断。在102例患者中,63例接受术后放疗,外照射靶区包括盆腔淋巴结引流区,瘤床区和阴道残端以及存在风险的其他淋巴结引流区。剂量约为45 Gy(常规分割1.8~2.0 Gy/次),对小体积可见未切除淋巴结加量10~15 Gy;有高危阴道残端复发风险的患者接受近距离放疗加量12 Gy/2次,不与外照射同日进行。接受化疗52例,化疗以铂类为基础。疗效指标评价包括总生存(overall survival, OS)和无病生存(disease-free survival, DFS)。
|
|
表 1 102例宫颈癌患者临床资料 Table 1 Characteristics of 102 patients with cervical cancer |
2.免疫组织化学及软件分析:免疫组织化学标准方法对宫颈癌组织芯片进行染色[5]。免疫组织化学分析软件Image-pro plus 6.0对PDK1、p-PDH和PKM2在宫颈癌组织中的染色结果进行定量评估,选取相同的棕黄色作为判断所有照片阳性的统一标准,对每张照片进行分析。按文献[6]方法,用照片阳性的累积吸光度(A)值除以组织的像素面积,计算得出平均吸光度(A)值,作为PDK1、p-PDH和PKM2在宫颈癌组织中的相对表达量。X-tile 2.0软件计算PDK1、p-PDH及PKM2与预后相关最佳cut-off值。
3. GEO数据集验证:GEO数据库下载文件名为GSE44001的数据集,数据集信息包含300例宫颈癌患者mRNA水平测序结果及患者的DFS时间。对应平台GPL14951的相关信息,获取PDK1、PDH和PKM2在mRNA水平的表达量。X-tile 2.0软件计算cut-off值,将PDK1、p-PDH和PKM2划分为高低表达两组。
4.统计学处理:GraphPad Prism 7.0和SPSS 17.0.1软件用于统计学分析。组间比较用χ2检验或Fisher精确概率法,生存分析采用Kaplan-Meier法,Log-rank检验比较组间生存率的差异,运用COX回归模型进行单因素及多因素预后分析。P < 0.05为差异有统计学意义。
结果1.复发、转移及生存状况:102例患者随访截止时间为2015年,失访7例,中位随访时间为73个月。复发转移28例(局部复发9例,远处转移19例),包含术后放疗复发转移14例(局部复发8例,远处转移6例)。102例患者5年OS率和DFS率分别为79.9%和76.1%。ⅠA/B、ⅡA和ⅡB期患者5年OS率分别为87.3%、82.7%和42.7%(χ2=14.048,P < 0.05),5年DFS率分别为85.5%、74.4%和41.8%(χ2=12.510,P < 0.05)。
2. PDK1、p-PDH及PKM2的表达与患者临床特征相关性:X-tile 2.0软件计算cut-off值后PDK1high、p-PDHhigh、PKM2high和PDK1high/p-PDHhigh/PKM2high组分别有49、34、58和13例患者,PDK1、p-PDH及PKM2高低表达免疫组织化学染色结果如图 1,相关性分析结果列于表 2。由表 2可知,PDK1high、p-PDHhigh、PKM2high及PDK1high/p-PDHhigh/PKM2high表达与患者年龄、分型和分期均无相关性(P>0.05)。p-PDH和PKM2的表达与盆腔淋巴结转移无相关性(P>0.05);PDK1high和PDK1high/p-PDHhigh/PKM2high患者更易发生盆腔淋巴结转移(χ2=10.890、7.407,P < 0.05)。
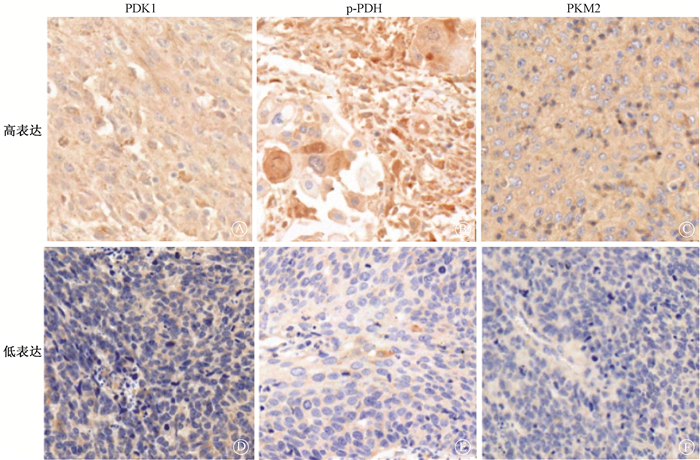
|
免疫组织化学染色×400 图 1 宫颈癌原发灶组织中PDK1、p-PDH及PKM2的表达 Immunohistochemical staining ×400 Figure 1 Expression of PDK1, p-PDH, and PKM2 in primary tissues of cervical cancer |
|
|
表 2 PDK1、p-PDH及PKM2的表达与临床特征的相关性 Table 2 Correlation between the expression of PDK1, p-PDH, PKM2 and the clinical characteristics |
3. PDK1、p-PDH及PKM2的表达对患者预后的影响:PDK1、p-PDH及PKM2高低表达组的OS和DFS曲线分离,但只有PDK1高低表达组的OS(χ2=4.875,P < 0.05)、PDK1high/p-PDHhigh/PKM2high与其他组的OS和DFS比较,差异有统计学意义(χ2=10.411、8.068,P < 0.05,图 2,3)。
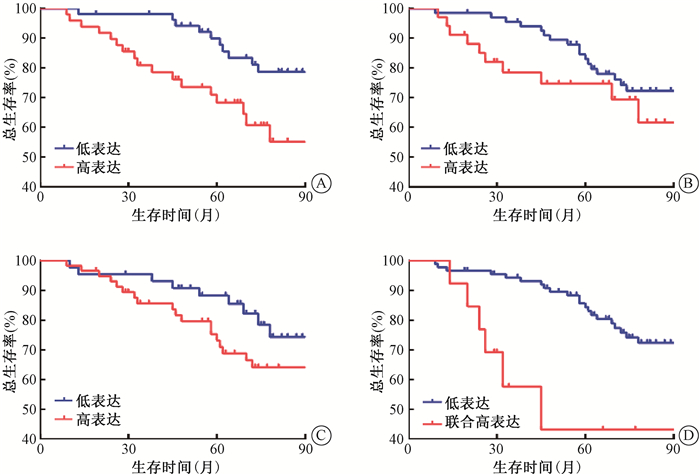
|
注:PDK1高表达组与低表达组比较,χ2=4.875,P < 0.05;PDK1、p-PDH和PKM2联合高表达与低表达比较,χ2=10.411,P < 0.05 图 2 102例患者不同分组总生存曲线比较A. PDK1;B. p-PDH;C. PKM2;D. PDK1、p- PDH和PKM2联合 Figure 2 Comparison of OS curves of 102 patients in different groups A. The expression of PDK1; B. The expression of p-PDH; C. The expression of PKM2; D. The co-expression of PDK1, p-PDH and PKM2 |
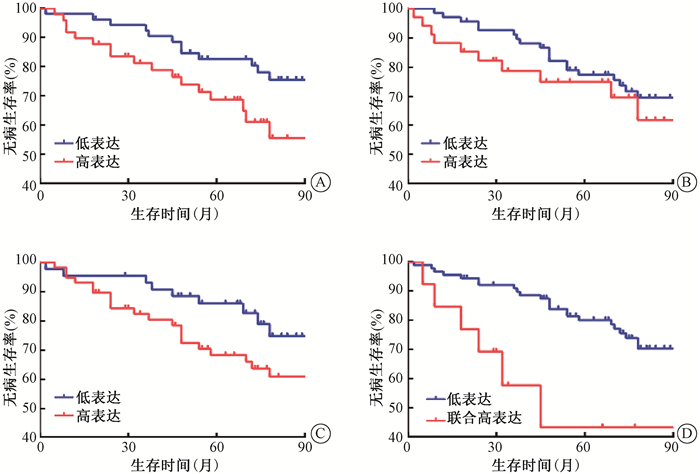
|
注:PDK1、p-PDH和PKM2联合高表达与低表达比较,χ2=8.068,P < 0.05 图 3 102例患者不同分组无病生存曲线比较A. PDK1;B. p-PDH;C. PKM2;D. PDK1、p-PDH和PKM2联合 Figure 3 Comparison of DFS curves of 102 patients in different groups A. PDK1 expression; B. p- PDH expression; C. PKM2 expression; D. co-expression of PDK1, p-PDH and PKM2 |
4.单因素和多因素分析结果:PDK1、PDK1high/p-PDHhigh/PKM2high、FIGO分期、盆腔淋巴结转移和术后放疗是OS的影响因素(P < 0.05),而K1high/p-PDHhigh/PKM2high、FIGO分期、盆腔淋巴结转移和术后放疗是DFS的影响因素(P < 0.05,表 3)。多因素分析显示,PDK1high/p-PDHhigh/PKM2high、FIGO分期和术后放疗是影响OS和DFS的独立预后因素(P < 0.05,表 4)。
|
|
表 3 102例宫颈癌患者预后单因素分析 Table 3 Univariate analysis of prognosis in 102 patients with cervical cancer |
|
|
表 4 102例宫颈癌患者预后多因素分析 Table 4 Multivariate analysis of prognosis in 102 patients with cervical cancer |
5. GEO数据集验证:在300例宫颈癌患者中,mRNA水平PDK1high、PDH高表达(PDHhigh)、PKM2high和三分子联合高表达(PDK1high/PDHhigh/PKM2high)组分别有132、105、99和24例。300例患者5年DFS率为85.1%。PDK1、PDH和PKM2高低表达组及PDK1high/PDHhigh/PKM2high和其他组的DFS曲线分离,差异有统计学意义(χ2=4.213、6.858、4.042、9.209,P < 0.05,图 4)。单因素分析显示,PDK1high、PDHhigh、PKM2high和PDK1high/PDHhigh/PKM2high是DFS的危险因素(P < 0.05)。数据库未提供分期及总生存数据,但DFS数据分析结果验证了三分子标志物蛋白高表达对宫颈癌的不良预后作用。
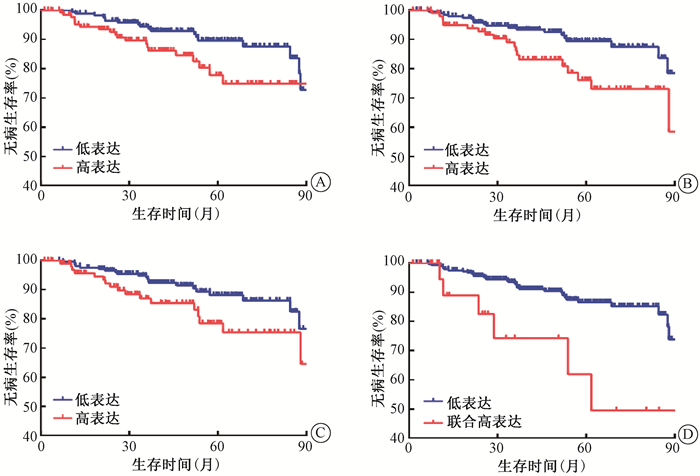
|
注:与低表达相比,χ2=4.213、6.858、4.042、9.209,P < 0.05 图 4 mRNA水平不同分组无病生存曲线比较A. PDK1;B. PDH;C. PKM2;D. PDK1、PDH和PKM2联合 Figure 4 Comparison of the DFS curves in different groups of mRNA levels A. PDK1 expression; B. PDH expression; C. PKM2 expression; D. Co-expression of PDK1, PDH and PKM2 |
6. PDK1high/p-PDHhigh/PKM2high的表达对患者术后放疗疗效的影响:63例术后放疗患者5年DFS率为76.8%,IA/B、ⅡA和ⅡB期患者5年DFS率分别为87.7%、74.6%和58.9%(P>0.05)。术后放疗患者中,PDK1、p-PDH、PKM2和PDK1high/p-PDHhigh/PKM2high组分别有35、23、33和9例,PDK1及p-PDH高低表达组DFS曲线分离,但差异无统计学意义(P>0.05);PKM2高低表达组,PDK1high/p-PDHhigh/PKM2high和其他组DFS曲线分离,差异有统计学意义(χ2=5.221、17.433,P < 0.05,图 5)。单因素分析显示,病理分型、盆腔淋巴结转移、PKM2及PDK1high/p-PDHhigh/PKM2high是术后放疗DFS的影响因素(表 5)。多因素分析显示,病理分型(HR=0.283,95% CI 0.091~0.882,P=0.030)和PDK1high/p-PDHhigh/PKM2high(HR=4.853,95% CI 1.543~15.265,P=0.007)是影响术后放疗DFS的独立预后因素。
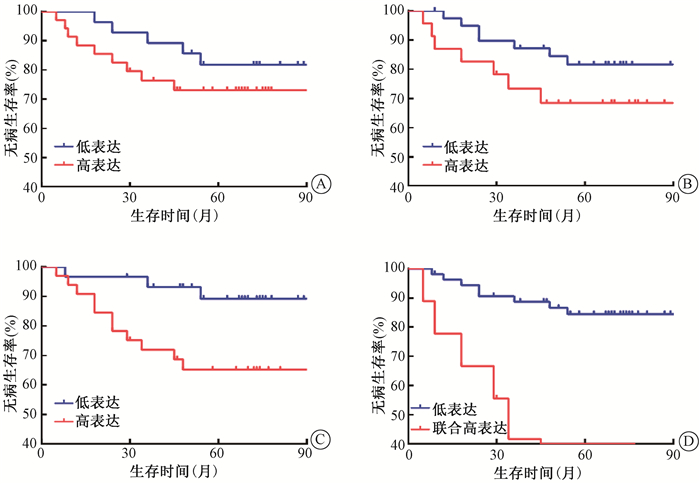
|
注:与低表达比较,PKM2高表达χ2=5.221,P < 0.05;PDK1/p-PDH/PKM2联合高表达χ2=17.433,P < 0.05 图 5 63例术后放疗患者不同分组无病生存曲线比较A. PDK1;B. p-PDH;C. PKM2;D. PDK1、p-PDH和PKM联合 Figure 5 Comparison of the DFS curves of 63 patients treated with postoperative radiation in different groups A. PDK1 expression; B. p-PDH expression; C. PKM2 expression; D. co-expression of PDK1, p-PDH and PKM2 |
|
|
表 5 63例术后放疗患者无病生存单因素分析 Table 5 Univariate analysis of the DFS in 63 patients treated with postoperative radiation |
讨论
宫颈癌作为最常见的女性生殖系统恶性肿瘤,FIGO分期是决定宫颈癌患者生存预后及治疗决策选择的主要因素,但部分患者临床分期相同,根据术后病理因素,按照Sedlis标准进行术后放化疗,临床预后却显著不同。因此,寻找新的指导宫颈癌预后及放疗疗效的预测指标,有助于弥补FIGO分期和Sedlis标准的不足,对临床分层治疗决策至关重要。本研究提出一种基于Warburg效应通路的分子分型,对判断宫颈癌患者的预后及术后放疗疗效有较好的参考价值。
Wigfield等[7]研究发现,表达PDK1的头颈鳞状细胞癌患者复发率增高,PDK1高低表达患者5年生存率分别为46.4%和70%。PDH阳性表达的胃癌患者5年生存率比阴性患者高37.8%,PDH是胃癌预后的独立影响因素[8]。p-PDH作为PDH的非活性形式,起着促进Warburg效应的作用[9]。在初次接受放疗的晚期局部宫颈癌鳞癌患者中,放疗抗拒患者PKM2过表达,PKM2高低表达患者5年无进展生存率分别为60.5%和80.4%,PKM2促进晚期局部宫颈鳞癌的放疗抵抗,是影响预后的独立危险因素[10]。本研究发现,PKM2是影响Ⅰ~ⅡB期宫颈癌术后放疗无复发生存的因素,PDK1是OS的影响因素,但多因素分析结果显示,单独高表达的PDK1、p-PDH和PKM2均不是患者预后的独立影响因素,可能与本研究样本量小有关。在含300例宫颈癌患者的GEO数据集验证分析中发现,单独分子高表达是DFS的危险因素。因此,单独分子高表达对宫颈癌的预后预测价值尚不能否定,有必要在更大样本中进行蛋白水平的研究。
为进一步明确Warburg效应通路中的分子对宫颈癌的预后价值,本研究联合三分子同时高表达分析发现,PDK1high/p-PDHhigh/PKM2high与盆腔淋巴结转移正相关。单因素和多因素分析结果一致表明,PDK1high/p-PDHhigh/PKM2high是影响Ⅰ~ⅡB期宫颈癌预后的独立危险因素。GEO数据集分析结果显示,三分子同时高表达患者无病生存预后最差。这可能与PDK1、p-PDH和PKM2协同增强Warburg效应有关,Warburg效应不仅能够维持肿瘤细胞快速增殖所需的大分子物质[11],同时Warburg效应中相关基因的突变,可通过诱导组蛋白和DNA的甲基化改变阻止细胞的分化,也可通过抑制脯氨酰羟化酶促进肿瘤细胞的扩散[12]。Warburg效应产生大量的乳酸堆积,能够增强肿瘤细胞的放射抵抗[13],进一步分析PDK1high/p-PDHhigh/PKM2high对术后放疗疗效的影响,单因素和多因素结果一致表明,PDK1high/p-PDHhigh/PKM2high是Ⅰ~ⅡB期宫颈癌术后放疗无病生存预后的独立危险因素。三分子同时高表达可协同促进肿瘤细胞的快速增殖,同时过度增强的Warburg效应能够增强肿瘤细胞的放疗抵抗性,这可能是三分子同时高表达患者预后差、疗效差的主要原因,但其具体机制仍有待进一步研究。
综上所述,PDK1high/p-PDHhigh/PKM2high有望作为一种新的宫颈癌分子分型,此分型对Ⅰ~ⅡB期宫颈癌患者的预后及放疗疗效评估有重要指导意义,为宫颈癌的分层治疗提供了新思路。同时本研究也存在一定的局限性,由于蛋白质芯片样本量有限,特别是对放疗患者,本研究结果可能存在偏差,尚待进一步临床验证。
利益冲突 无
作者贡献声明 厉娜负责芯片研究和数据分析,撰写论文;徐会负责修改论文;王巧丽负责标本收集构建芯片;邱惠、孙雪花负责协助处理数据分析;周云峰、周福祥负责研究设计和论文修改
| [1] |
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI:10.3322/caac.21492 |
| [2] |
Otto AM. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism[J]. Cancer Metab, 2016, 4: 5. DOI:10.1186/s40170-016-0145-9 |
| [3] |
Lin Y, Zhai H, Ouyang Y, et al. Knockdown of PKM2 enhances radiosensitivity of cervical cancer cells[J]. Cancer Cell Int, 2019, 19: 129. DOI:10.1186/s12935-019-0845-7 |
| [4] |
Meijer T, Peeters W, Dubois LJ, et al. Targeting glucose and glutamine metabolism combined with radiation therapy in non-small cell lung cancer[J]. Lung Cancer, 2018, 126: 32-40. DOI:10.1016/j.lungcan.2018.10.016 |
| [5] |
Liu T, Yin H. PDK1 promotes tumor cell proliferation and migration by enhancing the Warburg effect in non-small cell lung cancer[J]. Oncol Rep, 2017, 37(1): 193-200. DOI:10.3892/or.2016.5253 |
| [6] |
Yang Q, Liu Y, Huang Y, et al. Expression of COX-2, CD44v6 and CD147 and relationship with invasion and lymph node metastasis in hypopharyngeal squamous cell carcinoma[J]. PLoS One, 2013, 8(9): e71048. DOI:10.1371/journal.pone.0071048 |
| [7] |
Wigfield SM, Winter SC, Giatromanolaki A, et al. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer[J]. Br J Cancer, 2008, 98(12): 1975-1984. DOI:10.1038/sj.bjc.6604356 |
| [8] |
Sun XR, Sun Z, Zhu Z, et al. Expression of pyruvate dehydrogenase is an independent prognostic marker in gastric cancer[J]. World J Gastroenterol, 2015, 21(17): 5336-5344. DOI:10.3748/wjg.v21.i17.5336 |
| [9] |
Sale GJ, Randle PJ. Analysis of site occupancies in[32P]phosphorylated pyruvate dehydrogenase complexes by aspartyl-prolyl cleavage of tryptic phosphopeptides[J]. Eur J Biochem, 1981, 120(3): 535-540. DOI:10.1111/j.1432-1033.1981.tb05733.x |
| [10] |
Zhao Y, Shen L, Chen X, et al. High expression of PKM2 as a poor prognosis indicator is associated with radiation resistance in cervical cancer[J]. Histol Histopathol, 2015, 30(11): 1313-1320. DOI:10.14670/HH-11-627 |
| [11] |
Lunt SY, Vander HM. Aerobic glycolysis:meeting the metabolic requirements of cell proliferation[J]. Ann Rev Cell Dev Biol, 2011, 27: 441-464. DOI:10.1146/annurev-cellbio-092910-154237 |
| [12] |
Ward PS, Thompson CB. Metabolic reprogramming:a cancer hallmark even warburg did not anticipate[J]. Cancer Cell, 2012, 21(3): 297-308. DOI:10.1016/j.ccr.2012.02.014 |
| [13] |
Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate:a metabolic key player in cancer[J]. Cancer Res, 2011, 71(22): 6921-6925. DOI:10.1158/0008-5472.CAN-11-1457 |
 2020, Vol. 40
2020, Vol. 40


