结外NK/T细胞淋巴瘤(extranodal NK/T-cell lymphoma, ENKTCL)是一类起源于成熟NK细胞或T细胞的非霍奇金淋巴瘤(non-Hodgkin lymphoma,NHL)[1]。ENKTCL分布有明显的地域差异,中亚及南美地区发病率高,占NHL的10%左右;而欧洲和北美地区少见,发病率不足NHL的1%[2-5]。本病好发于中青年男性,早期疾病占70%~90%,鼻腔和韦氏环等上呼吸消化道是最常见的原发部位。
由于ENKTCL发病率较低且有明显的地域差异,因此目前关于ENKTCL治疗的数据大多来自于中国、韩国和日本等ENKTCL高发病的亚洲国家。早期研究证实单纯放疗治疗早期ENKTCL的完全缓解率为70%~100%,5年总生存(overall survival, OS)率在60%以上[6-9]。但30%~50%患者治疗后出现疾病复发或转移,其中远地播散是主要的治疗失败模式[10]。因此,单纯放疗对于早期ENKTCL的治疗可能是不够的,需全身化疗减少疾病远处播散。已有多项小样本前瞻性Ⅱ期临床试验证实,同步、序贯或夹心放化疗综合治疗在早期ENKTCL患者中取得了较好的疗效,5年OS率达66%~90%,不低于或优于单纯放疗的历史对照[11-17]。因此,目前基于中国在内的亚洲国家的数据,放疗或放化疗综合治疗认为是早期ENKTCL重要的治疗手段。然而,上述数据多来自于小样本的回顾性研究或前瞻性单臂研究,目前尚无关于西方ENKTCL患者人群的治疗模式的大样本的对照研究数据。本研究拟基于SEER数据库的西方ENKTCL患者的临床及生存资料,验证上述治疗模式在早期ENKTCL患者中的有效性。
资料与方法1.资料选取:利用美国监测、流行病学及预后数据库(surveillance, epidemiology and end results,SEER),通过SEER*Stat软件(SEER*Stat 8.3.6)获取2000—2016年登记的ENKTCL患者临床病理及生存资料。疾病分类参照第3版国际肿瘤疾病分类(ICD)方法,采用ICD-O-3-9719作为NKTCL的疾病编码。
2.入组及排除标准:按照纳入和排除标准筛选符合条件的ENKTCL患者。纳入标准:病理诊断为ENKTCL且不合并其他肿瘤者;Ⅰ/Ⅱ期;有随访结果和存活状态记录;患者年龄、种族、性别、肿瘤原发部位、分期等信息明确。排除标准:原发结内NKTCL者;进展期(Ⅲ/Ⅳ);原发部位、分期信息不明确或无记录者;未接受治疗或无治疗记录者。
3.信息收集:收集患者的临床病理及生存信息,包括登记号、诊断时间、诊断年龄、性别、种族、婚姻状况、原发部位、组织类型、病理分级、细胞来源、分期、治疗情况、随访时间、生存情况、死亡原因等数据。根据SEER数据库,种族记录分为白种人、黑种人和其他人种(印第安人/阿拉斯加本地人和亚太人群)。疾病分期按照Ann Arbor分期系统。恶性细胞来源分NK细胞、T细胞来源或其他。B症状包括发热、盗汗和(或)体重减轻。根据放化疗记录将患者分为单纯化疗、单纯放疗及放化疗联合治疗组。
4.统计学处理:本研究主要研究目标为全因死亡。分类资料构成比的比较采用χ2检验。生存分析采用Kaplan-Meier法,组间生存率的单因素分析采用Log-rank检验,多因素分析采用Cox风险回归模型。倾向评分匹配(propensity-score matched,PSM)采用1:1匹配方法,匹配容差为0.02,均衡放疗组和放化疗组患者的差异性预后特征,以期减少回顾性研究的选择偏倚。所有数据分析采用SPSS 24.0。P<0.05认为差异有统计学意义。生存曲线做图由Graphpad Prism 8.0.1软件完成。
结果1.患者基线特征:2000—2016年SEER数据库共登记了944例诊断明确的ENKTCL的患者信息。根据纳入及排除标准,共计448例患者纳入本研究,其中108例患者接受单纯化疗,100例患者接受单纯放疗,240例患者接受放化疗综合治疗。
患者基线特征和各亚组生存数据见表 1。总体患者的中位诊断年龄为52(8~93)岁,男女比例为1.8 :1。绝大多数疾病为NK细胞来源(73.7%)。原发上呼吸消化道病变占88.6%。此外,年龄>60岁、Ⅱ期和伴随B症状患者分别占总人群的33.9%、31.3%和35.9%。
|
|
表 1 总体ENKTCL患者基线特征及生存预后的单因素分析结果 Table 1 Baseline characteristics and monovariate analysis of survival for overall patients with ENKTCL |
2.生存及预后分析:结果示于图 1。由图 1可见,总体人群的中位OS时间为59.0个月,5年OS率为49.0%;接受放化疗综合治疗、单纯放疗及单纯化疗的患者5年OS率分别为62.1%、41.5%和28.5% (χ2=41.727, P < 0.001)。单因素分析列于表 1,提示年龄>60岁、Ⅱ期病变、原发上呼吸消化道以外病变、伴随B症状者及接受单纯化疗的患者生存较差。将上述因素带入Cox回归模型,分析结果列于表 2,显示年龄、分期、肿瘤原发部位及治疗模式是影响OS的预后因素。
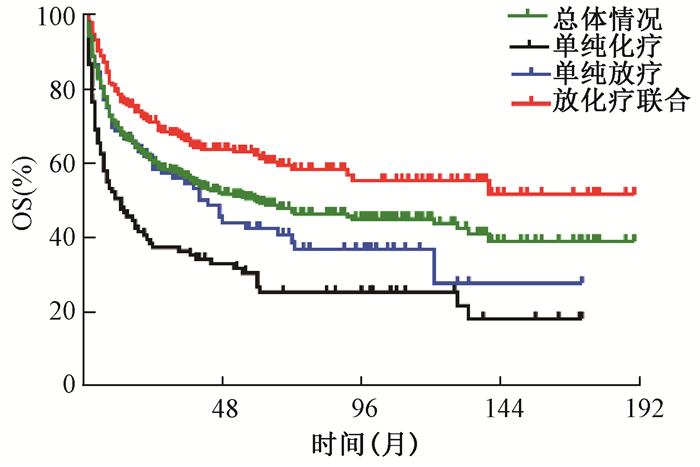
|
注:OS.总生存;ENKTCL.结外NK/T细胞淋巴瘤 图 1 ENKTCL患者的总生存曲线 Figure 1 OS curves of ENKTCL patients |
|
|
表 2 ENKTCL患者生存预后多因素分析结果 Table 2 Multivariate analysis of survival for patients with ENKTCL |
3.放疗显著改善早期患者生存:结果示于图 2。由图 2可见,放疗明显改善了患者总生存。与单纯化疗相比,接受单纯放疗的患者的5年OS率显著提高(41.5% vs. 28.5%,HR 0.673,95% CI:0.476~0.952, P=0.022)。接受放疗(放疗±化疗)的患者5年OS率明显高于未接受放疗(单化疗)的患者(55.9% vs.28.5%, HR 0.408, 95%CI:0.334~0.640, χ2=10.823, P < 0.001)。而化疗并未显示出类似的生存获益:接受化疗(化疗±放疗)的患者与未接受化疗(单纯放疗)的患者5年OS率的差异无统计学意义(51.2% vs. 50.6%, HR 0.809, 95% CI:0.597~1.097, P=0.167)。多因素分析结果列于表 2,显示对比单纯化疗,包含放疗的治疗模式(单纯放疗或放化疗综合治疗)明显提高了早期ENKTCL患者的生存,降低了死亡风险。
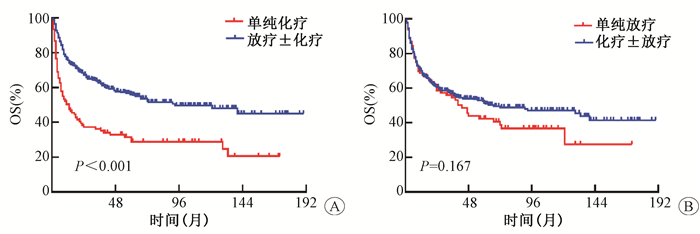
|
注:OS.总生存 图 2 放疗明显改善早期ENKTCL患者总生存 A.放疗±化疗对比单纯化疗对OS的影响;B.化疗±放疗对比单纯放疗对OS的影响 Figure 2 Radiation significantly improved the survival of patients with early stage ENKTCL A. Comparison of OS between radiation±chemotherapy and chemotherapy alone; B. Comparison of OS between chemotherapy±radiation and radiation alone |
4.放疗基础上联合化疗进一步改善了早期患者生存:本研究比较了单纯放疗组(n=100)和放化疗综合治疗组(n=240)患者的生存情况。因单纯放疗组和放化疗组在患者年龄及疾病分期分布上不均衡(表 3),因此本研究采用(PSM)法均衡上述预后差异因素。两组各有90例患者完成配对,匹配后两组患者的各预后因素完全均衡(表 3)。由图 1可见,匹配前患者的生存分析显示相比于单纯放疗,接受放化疗综合治疗的患者5年OS率明显提高,死亡风险降低42.2% (HR 0.578, 95% CI:0.413~0.808, P=0.001)。多因素分析也进一步证实了上述结果(HR 0.600, 95% CI:0.411~0.877, P=0.008)。匹配后ENKTCL患者生存分析见图 3,接受放化疗综合治疗者OS亦明显优于接受单纯放疗的患者(HR 0.572, 95% CI:0.369~0.885, P=0.012)。
|
|
表 3 倾向评分匹配前后单放疗组及放化疗综合治疗组患者临床特征匹配情况 Table 3 Comparison of characteristics between ENKTCL patients who received radiation alone and those with combined chemoradiotherapy before and after propensity-score match |
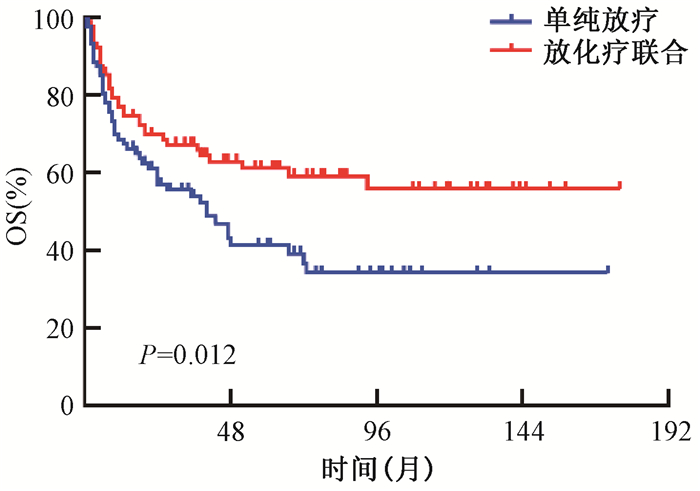
|
注:OS.总生存 图 3 倾向得分匹配后早期ENKTCL患者的总生存曲线 Figure 3 OS curves of patients with early stage ENKTCL after the propensity-score match |
讨论
与弥漫大B细胞淋巴瘤等常见的NHL不同,ENKTCL对化疗相对不敏感。既往多采用蒽环类药物为基础的方案化疗,疗效欠佳。随着多药耐药基因及P糖蛋白的发现,左旋门冬酰胺酶/培门冬酶、吉西他滨、铂类等非蒽环类药物逐步应用于ENKTCL的治疗并改善了患者的生存[18-22]。但早期ENKTCL接受单纯化疗的疗效欠佳,有效率为50%~70%,5年OS率仅为15%~35%,即使非蒽环类药物单纯化疗的5年OS率也只有40%左右[9, 23-26]。多项研究证实,放疗明显改善了ENKTCL的生存,5年OS率在60%以上,低危患者接受单纯放疗的5年OS率可达88.8%[5-7, 27-30]。与上述结论一致,本研究中单纯化疗患者的5年OS率仅为28.5%,明显低于单纯放疗或放化疗综合治疗者(41.5% vs. 62.1%,P < 0.001)。由此可见,ENKTCL对放疗高度敏感,放疗是早期患者治疗的基石。
早期小样本回顾性对照研究直接对比了单纯放疗和放化疗联合治疗早期ENKTCL的生存数据,结果显示对比单纯放疗,化疗的加入未能有效减少远地转移或局部复发的发生率,未能进一步提高生存率[6, 28-29, 31]。一项Meta分析纳入了21项研究的1 185例早期ENKTCL,结果提示单纯放疗和放化疗两者在治疗有效率(P=0.73)、无进展生存(P=0.21)及OS(P=0.21)的差异均无统计学意义;化疗的加入亦未能有效减少远地转移或局部复发的发生率,反而增加了治疗相关不良反应[29]。值得注意的是上述研究均为早期蒽环类药物化疗时代的数据,样本量小,且各研究中放疗技术、放射野及剂量、化放疗联合方式均不整齐,可能是造成此阴性结果的原因。
近期的多项亚洲国家的前瞻性研究证实放化疗综合治疗明显提高了早期ENKTCL患者的长期生存。同步、序贯或“三明治夹心”的放化疗综合治疗在早期患者中取到了良好的疗效,完全有效率在80%~95%,5年OS率在66%~90%,优于单纯放疗的历史对照[12-16]。此外,一项来自中国的大样本回顾性对照研究[9]也证实了放化疗综合治疗优于单纯放疗,改善早期ENKTCL的长期生存。该研究分析了全国10个中心的1 273例早期初治ENKTCL患者的不同治疗及生存数据,其中170例患者接受单纯化疗,253例患者接受单纯放疗,850例患者接受放化疗综合治疗[9]。生存分析显示,与单纯化疗相比,单纯放疗或放化疗综合治疗明显改善了患者总生存(5年OS率,69.6% vs. 67.7% vs. 33.9%, P < 0.001),进一步分析显示相较于单纯放疗,放化疗联合治疗明显改善了高危患者的总生存(5年OS率,72.2% vs.51.7%, P=0.017)[9]。此外,另一项墨西哥的前瞻性研究也证实了放化疗综合治疗优于单纯放疗或单纯化疗,改善了早期ENKTCL患者长期生存(5年OS率, 86.0% vs. 64.0% vs. 45.0%, P < 0.001)[32]。本研究于西方ENKTCL患者人群中验证了不同治疗模式对预后的影响,分析结果与上述研究一致:接受放化疗综合治疗的患者5年OS率明显高于单纯放疗或单纯化疗(62.1% vs. 41.5% vs. 28.5%, P < 0.001),且放化疗联合治疗的生存优势在匹配后的患者中仍存在。因此,放化疗综合治疗是ENKTCL患者的最佳治疗模式。
本研究存在以下不足:首先,尽管本研究样本量较大,但作为回顾性研究仍存在选择偏倚,分析中已用PSM方法尽量减小了该偏倚。其次,一些较重要的预后指标,如东部癌症协作组(ECOG)评分、血清乳酸脱氢酶(LDH)水平、病毒(EBV)-DNA拷贝数等数据在SEER数据库中并没有详细有效记录,无法分析上述因素对生存的影响,也无法较准确对不同治疗组优势获益人群进行筛选。最后,患者的局部复发、远地播散、放疗剂量、化疗方案及不良反应数据在SEER数据库中缺失,无法分析不同治疗策略的失败模式、无进展生存及安全性。因此,放化疗综合治疗模式的优势需要更加严谨的随机对照研究数据进一步证实。
利益冲突 所有作者均声明本文不存在任何利益冲突
作者贡献声明 齐菲分析数据和撰写论文;柴玥、韦雨策负责数据采集;董梅负责选题设计
| [1] |
Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127(20): 2375-2390. DOI:10.1182/blood-2016-01-643569 |
| [2] |
Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States[J]. Br J Haematol, 2014, 164(4): 536-545. DOI:10.1111/bjh.12659 |
| [3] |
Sun J, Yang Q, Lu Z, et al. Distribution of lymphoid neoplasms in China:analysis of 4, 638 cases according to the World Health Organization classification[J]. Am J Clin Pathol, 2012, 138(3): 429-434. DOI:10.1309/AJCP7YLTQPUSDQ5C |
| [4] |
Haverkos BM, Pan Z, Gru AA, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT):an update on epidemiology, clinical presentation, and natural history in North American and European cases[J]. Curr Hematol Malig Rep, 2016, 11(6): 514-527. DOI:10.1007/s11899-016-0355-9 |
| [5] |
Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population[J]. Leuk Lymphoma, 2016, 57(11): 2575-2583. DOI:10.1080/10428194.2016.1180689 |
| [6] |
Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma[J]. J Clin Oncol, 2006, 24(1): 181-189. DOI:10.1200/JCO.2005.03.2573 |
| [7] |
Li YX, Wang H, Jin J, et al. Radiotherapy alone with curative intent in patients with stage I extranodal nasal-type NK/T-cell lymphoma[J]. Int J Radiat Oncol Biol Phys, 2012, 82(5): 1809-1815. DOI:10.1016/j.ijrobp.2010.10.040 |
| [8] |
Wang H, Li YX, Wang WH, et al. Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma[J]. Int J Radiat Oncol Biol Phys, 2012, 82(3): 1115-1121. DOI:10.1016/j.ijrobp.2011.02.039 |
| [9] |
Yang Y, Zhu Y, Cao JZ, et al. Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma:analysis from a multicenter study[J]. Blood, 2015, 126(12): 1424-1432. DOI:10.1182/blood-2015-04-639336 |
| [10] |
Li YX, Liu QF, Wang WH, et al. Failure patterns and clinical implications in early stage nasal natural killer/T-cell lymphoma treated with primary radiotherapy[J]. Cancer, 2011, 117(22): 5203-5211. DOI:10.1002/cncr.26167 |
| [11] |
Tsai HJ, Lin SF, Chen CC, et al. Long-term results of a phase Ⅱ trial with frontline concurrent chemoradiotherapy followed by consolidation chemotherapy for localized nasal natural killer/T-cell lymphoma[J]. Eur J Haematol, 2015, 94(2): 130-137. DOI:10.1111/ejh.12405 |
| [12] |
Qi F, Wang WH, He XH, et al. Phase 2 study of first-line intensity modulated radiation therapy followed by gemcitabine, dexamethasone, and cisplatin for high-risk, early stage extranodal nasal-type NK/T-Cell Lymphoma:The GREEN Study[J]. Int J Radiat Oncol Biol Phys, 2018, 102(1): 61-70. DOI:10.1016/j.ijrobp.2018.05.046 |
| [13] |
Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of "sandwich" L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to ⅡE, nasal type, extranodal natural killer/T-cell lymphoma[J]. Cancer, 2012, 118(13): 3294-3301. DOI:10.1002/cncr.26629 |
| [14] |
Yamaguchi M, Tobinai K, Oguchi M, et al. Phase Ⅰ/Ⅱ study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma:Japan Clinical Oncology Group Study JCOG0211[J]. J Clin Oncol, 2009, 27(33): 5594-5600. DOI:10.1200/JCO.2009.23.8295 |
| [15] |
Yoon DH, Kim SJ, Jeong SH, et al. Phase Ⅱ trial of concurrent chemoradiotherapy with L-asparaginase and MIDLE chemotherapy for newly diagnosed stage Ⅰ/Ⅱ extranodal NK/T-cell lymphoma, nasal type (CISL-1008)[J]. Oncotarget, 2016, 7(51): 85584-85591. DOI:10.18632/oncotarget.11319 |
| [16] |
Kim SJ, Kim K, Kim BS, et al. Phase Ⅱ trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to ⅡE, nasal, extranodal NK/T-cell lymphoma:consortium for improving survival of lymphoma study[J]. J Clin Oncol, 2009, 27(35): 6027-6032. DOI:10.1200/JCO.2009.23.8592 |
| [17] |
Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T-cell lymphoma:analysis of safety and efficacy from the Asia Lymphoma Study Group[J]. Blood, 2012, 120(15): 2973-2980. DOI:10.1182/blood-2012-05-431460 |
| [18] |
Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type[J]. Blood, 2018, 131(23): 2528-2540. DOI:10.1182/blood-2017-12-791418 |
| [19] |
Yamaguchi M, Miyazaki K. Current treatment approaches for NK/T-cell lymphoma[J]. J Clin Exp Hematop, 2017, 57(3): 98-108. DOI:10.3960/jslrt.17018 |
| [20] |
Tse E, Kwong YL. How I treat NK/T-cell lymphomas[J]. Blood, 2013, 121(25): 4997-5005. DOI:10.1182/blood-2013-01-453233 |
| [21] |
Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation:is it optimal for localized nasal natural killer/T-cell lymphoma?[J]. Ann Oncol, 2001, 12(3): 349-352. DOI:10.1023/a:1011144911781 |
| [22] |
Wang L, Xia ZJ, Huang HQ, et al. Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) in the treatment of stage IE/Ⅱ E extranodal natural killer/T cell lymphoma, nasal type:13-year follow-up in 135 patients[J]. Int J Hematol, 2012, 96(5): 617-623. DOI:10.1007/s12185-012-1174-y |
| [23] |
Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma:a study of 136 cases from the International Peripheral T-Cell Lymphoma Project[J]. Blood, 2009, 113(17): 3931-3937. DOI:10.1182/blood-2008-10-185256 |
| [24] |
Fang H, Jin J, Wang WH, et al. Prognostic factors and treatment outcomes for patients with stage Ⅱ extranodal nasal-type natural killer/T-cell lymphoma of the upper aerodigestive tract[J]. Leuk Lymphoma, 2014, 55(8): 1832-1837. DOI:10.3109/10428194.2013.852668 |
| [25] |
You JY, Chi KH, Yang MH, et al. Radiation therapy versus chemotherapy as initial treatment for localized nasal natural killer (NK)/T-cell lymphoma:a single institute survey in Taiwan[J]. Ann Oncol, 2004, 15(4): 618-625. DOI:10.1093/annonc/mdh143 |
| [26] |
Li CC, Tien HF, Tang JL, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma[J]. Cancer, 2004, 100(2): 366-375. DOI:10.1002/cncr.11908 |
| [27] |
Li YX, Fang H, Liu QF, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring[J]. Blood, 2008, 112(8): 3057-3064. DOI:10.1182/blood-2008-05-160176 |
| [28] |
Kim TH, Kim JS, Suh YG, et al. The roles of radiotherapy and chemotherapy in the era of multimodal treatment for early-stage nasal-type extranodal natural killer/T-cell lymphoma[J]. Yonsei Med J, 2016, 57(4): 846-854. DOI:10.3349/ymj.2016.57.4.846 |
| [29] |
Jiang L, Li SJ, Jiang YM, et al. The significance of combining radiotherapy with chemotherapy for early stage extranodal natural killer/T-cell lymphoma, nasal type:a systematic review and meta-analysis[J]. Leuk Lymphoma, 2014, 55(5): 1038-1048. DOI:10.3109/10428194.2013.827789 |
| [30] |
Deng T, Zhang C, Zhang X, et al. Treatment outcome of radiotherapy alone versus radiochemotherapy in IE/ⅡE extranodal nasal-type natural killer/T cell lymphoma:a meta-analysis[J]. PLoS One, 2014, 9(9): e106577. DOI:10.1371/journal.pone.0106577 |
| [31] |
Khan L, Hodgson D, Sun A, et al. A single institution experience of extranodal natural killer/T cell lymphoma of nasal type[J]. Leuk Lymphoma, 2015, 56(1): 80-84. DOI:10.3109/10428194.2014.909039 |
| [32] |
Avilés A, Neri N, Fernández R, et al. Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma:results of a clinical trial[J]. Med Oncol, 2013, 30(3): 637. DOI:10.1007/s12032-013-0637-1 |
 2020, Vol. 40
2020, Vol. 40


