2. MHC Key Lab of Radiation Biology, School of Public Health, Jilin University, Changchun, Jilin 130021, China;
3. Shenzhen Haibo Technology Co., Ltd., Shenzhen 518057, China
When we refer to radiation medicine, at least three components are included, i.e., radiobiology, medical physics and clinical radiation oncology. With the advances in medical physics in computing technology, the precision radiotherapy (RT) becomes possible; the outcomes of radiotherapy have also improved during the last decade. While the development of radiobiology theory seems to lag behind the clinical application of RT, for examples, the underlying mechanisms for killing and dose-effect models need to be updated, which limits the further optimization of treatment strategies. In this article, the principles of radiobiology and the challenges derived from modern RT are going to be addressed, the solution of these theoretical questions will contribute to the progress of RT and ultimately benefit cancer patients.
1 The precision radiotherapy: principle and applicationX-rays were discovered by the German physicist Roentgen in 1895 while studying cathode rays in a gas discharge tube[1]. Since then, the constant technological advances have contributed to the development of RT. RT alone or, more commonly, combined with surgery and drugs, has become a major treatment for cancers. Since RT is recommended as a vital component of curative management as well as a successful, well-tolerated, time-efficient and cost-effective intervention in palliative oncology care[2], the primary principle of RT lies in precise localization of sufficient dose in the target lesion while minimizing the damage to the surrounding normal tissues. When RT was firstly applied into cancer patients last century, its harmful effect was apparent[3], nowadays such high-precision radiotherapies as intensity modulated radiotherapy (IMRT) and stereotactic radiotherapy(SRT) have improved treatment outcomes in cancers and RT has becoming a standard curative treatment.
Since the launch of a new "Precision Medicine Initiative" in the United States on January 20, 2015, precision medicine has gained attentions. In radiation oncology, the emergence and development of SRT, IMRT and 3D-CRT has opened a new era of precision RT[4-6]. Under this circumstance the tumor is treated with radiation combined with computer network technology, physics and other technologies, throughout the entire RT process. Precision RT is characterized as follows: the target lesion has the maximum dose, the surrounding normal tissues have the smallest dose, the dose distributed uniformly in the target area as well as the accurate target positioning and radiation delivery. Compared with traditional RT, the advantages of precision RT are high precision, high dose, high efficacy and low damage. As a precision RT strategy, SRT requires a multidisciplinary approach combining radiation oncology, medical oncology, surgical oncology, interventional radiology, imaging and pathology[7]. SRT was considered a safe and feasible approach for early stage non-small lung cell cancer[8], locally advanced pancreatic cancer[9], localized prostate cancer[10], inoperable hepatocellular carcinoma[11] and recurrent unresectable head and neck cancer[12], the efficacy of SRT has also been shown for lung cancer patients who were previously treated with conventional radiotherapy[13]. Hypofractionation, a small number of larger dose fraction, has been normally used for SRT, disasters from the past involving unacceptable late normal tissue damage limit this strategy for years and it would be folly to espouse such strategies at the expense of either local cancer control or increased toxicity to normal tissues, though it′s more convenient for patients and reduce economic burden in healthcare systems.
In addition, precision RT should also be individualized, which is an ideal approach which could "tailor" treatment based on a large number of individuals′ clinical investigations, pathology and molecular profiles. We believe precision RT combined with individualized RT will become more and more important in the combined modality therapy of cancers.
2 Is radiosensitivity, the core concept of radiobiology, facing challenge?Radiobiology introduces the reactions of organisms to different radiations, including the physical and chemical characteristics of rays, the mode of actions, radiosensitivity, deterministic effect and stochastic effect, factors determining the radiation outcomes, molecular damage and cell death, "4Rs" theory for radiation oncology, rationale of fractionation, and so on.
The principle of RT is to kill cancer cells as much as it could, and to avoid the damage to adjacent normal cells at the same time. Theoretically, the more differential in radiosensitivity between cancer cells and normal cells, the better the treatment efficacy and the less side-effect. Radiosensitivity refers to the reaction of organisms to radiation, the faster and more intense the response, the higher the radiosensitivity is. Generally, cancers with lower radiosensitivity are not recommended for RT treatment.
With the development of modern RT technologies, both target delineation and radiation delivery have become more accurate, the local radiation intensity and the steep dose gradient could increase the deposition of radiation energy on cancer cells and simultaneously decrease the damage to adjacent normal cells. Multi-directional sources of rays could also disperse the dose deposition at the entry or exit point of dose. These make it possible to treat cancers with lower radiosensitivity. The increase in radiation deposition to local cancer region will broaden the application of RT in various cancers. Then the question arises, whether the radiation dose can be increased greatly? The answer is "no". Firstly, we have to think about the total tolerance of organs to cumulative doses, for example, for total liver, 2/3 liver, 1/3 liver, the tolerance dose is about 30, 45, 55 Gy, respectively. Secondly, target areas will inevitably cover adjacent normal cells to some extent, and the therapeutic ratio is still the concern even if the involved normal tissue region is getting smaller and smaller. Thirdly, the local radiation could trigger the interactions between cancer cells and microenvironment. Finally, we have to think about the non-target or abscopal effects of radiation, after all the higher dose means the higher risk of non-target effects. The non-target effects and the interactions between cancer cells and microenvironment will be mentioned later on.
The radiation dose we refer to is not only the cumulative dose, but also the biologically equivalent dose (BED). By different fractionation, the same cumulative dose could create different BED, i.e., the fewer the fractionations, the higher the BED, and the more killing to the cancer cells.
3 Is the traditional dose-effects model still suitable for modern RT?Radiation biology applied to clinical radiotherapy is concerned with the relationship between a given absorbed dose of radiation and the consequent biological response. The dose-response curves have a sigmoid shape, with the incidence tending tozero as dose tends to be zero and the incidence tending to 100% at very large doses, by which the different response between cancer and normal tissues could be differentiated. Survival curve elucidates the different mathematics models for different radiation rays and radiation doses.
3.1 The dose-response curve and the therapeutic ratio (TR)The ratio of the cancer response for a fixed level of normal tissue damage is called therapeutic ratio (TR). In the dose-response curves, TCP (tumor control probability) is plotted as a function of total dose, and the incidence of normal tissue complications is also plotted as a function of dose. Since the adjacent normal tissues are inevitably exposed to radiation, we have to think about the TR. The broader the distance between TCP and NTCP (normal tissue complication probability) curve, the greater the discrepancy of radiosensitivity between cancer and normal tissues, and the more possibilities the high dose of radiotherapy might be used. Moreover, under certain circumstance, the CTV and PTV could be controlled as smaller as close to GTV, especially for SRT, which also contribute to the increase of RT dose, and thus improve RT efficacy.
3.2 Survival curve and Linear-quadratic model (LQ)A survival curve describes the relationship between the radiation dose and the proportion of cells that survive. Survival curves for mammalian cells are presented in Figure 1. At low doses for sparsely ionizing (low LET) radiation such as X-rays, the survival curve starts out straight on the log-linear plot [HJ]with a finite initial slope, i.e., the surviving fraction is an exponential function of dose. At higher doses, the curve bends. This bending region extends over a dose range of a few Grays. At very high doses, the survival curve often tends to straighten again, the surviving fraction returns to being an exponential function of dose, which might generally not occur until doses in excess of those used as daily fractions in radiotherapy have been reached[14] (we have to lay emphasis on "generally", for recent years certain SRT treatment use up to 20 Gy per fraction). By contrast, for densely ionizing (high LET) radiations, such as α-particles or low-energy neutrons, the survival curve is a straight line from the origin, survival approximates to an exponential function of dose.

|
Figure 1 The survival curve shows the relationship between radiation dose and the survival cells |
The tool most commonly used for quantitative predictions of dose/fractionation dependencies in radiotherapy is the mechanistically based linear-quadratic (LQ) model. The LQ formalism is now almost universally used for calculating radiotherapeutic isoeffect doses for different fractionation schemes. Up to 2008 Brenner[15] believed no evidence of problems when the LQ model was applied in the clinic. There is an important point, the linear and quandratic contributions to cell killing are equal at a dose that is equal to the ratio of α to β(α/β). Based on the α/β, the medical physicists and radiation oncologists calculate the BED and design the RT strategies.
The linear-quadratic (LQ) model is widely used to model the effect of total dose and dose per fraction in conventionally fractionated radiotherapy. Much of the data used to generate the model are obtained in vitro at doses well below those used in radiosurgery. Then problems arise, by using hypofractionation RT or SRS, in which the single dose is approaching to 10-20 Gy, the linear-quadratic (LQ) model and biologically equivalent dose (BED) have been suggested to be incorrect when used for hypofractionation[16]. What′s the prerequisite between LQ and other models such as universal survival curve, LQL model, and the generalized LQ (gLQ) model [17-19], is the most important concern. The inaccurate assessment of BED will definitely affect the practice and the efficacy of RT. Additionally, the LQ model underestimates tumor control observed at radiosurgical doses, do not reflect the vascular and stromal damage produced at the high doses per fraction encountered in radiosurgery and ignore the impact of radioresistant subpopulations of cells. The appropriate modeling of both tumor control and normal tissue toxicity in radiosurgery requires the application of emerging understanding of molecular-, cellular-, and tissue-level effects of high-dose/fraction ionizing radiation and the role of cancer stem cells[20].
BED is used for the comparison with the effects of fractionation into the total values. Within the LQ model, BED is defined as (without or with repopulation):
| $ \begin{array}{l} {\rm{BED}} = {N_{\rm{d}}} \times \left[ {1 + \frac{d}{{\alpha /\beta }}} \right]{\rm{or}}\;{\rm{BED}} = {N_{\rm{d}}} \times \left[ {1 + \frac{d}{{\alpha /\beta }}} \right] - \\ \;\;\;\;\;\;\;\;\;\;\;K(T - {T_{{\rm{delay}}}}) \end{array} $ |
Where Nd and d represent the total dose and dose per fraction, respectively. The quantities α and β represent the linear and quadratic components of the cell-survival curve and their ratio is characteristic of the biologic effect for the tissue under consideration. Similarly, the normalized total dose (NTD) may be defined as the total dose delivered in conventional fractionation (e.g., 2 Gy/fraction) that corresponds to a particular BED. These biologic indices are useful to compare different fractionation schedules. However, one should be cautioned that use of the LQ model is only an approximation, and its application for hypofractionated SRT deliveries has yet to be fully verified[20]. Nevertheless, it is apparent that the dose prescriptions for SRT have substantially greater biologic effects.
3.3 The "4Rs" of radiobiology is restricted by dose rate effectsThe multifraction regimens commonly used in conventional therapy are largely a consequence of radiobiologic experiments in 1920s. For almost one century′s development, the "4Rs" theory has been established for the efficacy of fractionation and it can be summarized as: repair of sublethal damage, reassortment of cells within the cell cycle, repopulation and reoxygenation. That is, dividing a dose into several fractions spares normal tissues because of the repair of sublethal damage between dose fractions and repopulation of cells if the time is sufficiently long, at the same time, increases damage to cancer because of the reoxgenation and reassortment of cells into radiosensitive phases of cycle between dose fraction.
Nowadays, it has been recognized that the "four Rs" of radiobiology is restricted by dose rate and no Rs takes place for dose rate > 100 cGy/min (Figure 2). Repopulation occurs and increases with dose rate < 1.0 cGy/min, reoxygenation and redistribution occur and increase with dose rate < 10 cGy/min, repair occurs and increases with dose rate < 100 cGy/min, and 4Rs are not affected by dose rate < 0.01 cGy/min[21] (Figure 2). In clinical radiotherapy, the traditional pattern of high dose-rate for external beam therapy and low dose-rate for intracavitary and interstitial therapy has been challenged in recent years. Although the limitation of biological effects by dose rate, different radiation sources show different effects from "4Rs", for example, photon and proton treatment are highly dependent on cell cycle and oxygen concentration, while heavy ion treatment is less dependent on them, the "4Rs" limitation need further assessment.
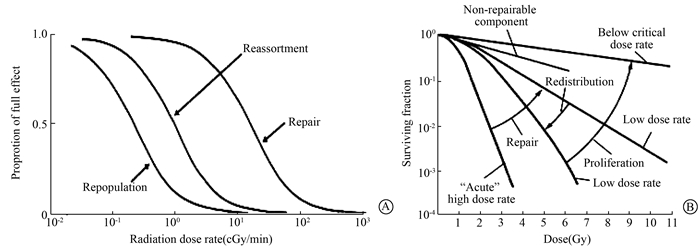
|
Figure 2 The effects of dose rate on the "four Rs" phenomena A. The range of dose rates over which repair, reassortment and reproliferation may influence radiation effects; B. The dose rate effect resulting from repair of sublethal damage, redistribution in the cycle and cell proliferation. The dose-response curve for acute exposure is characterized by a broad initial shoulder, as the dose is reduced, the cure becomes progressively shallower as the sublethal damage is repaired |
Shape of survival curve for mammalian cell surviving is plotted logarithmic scale. For sparsely ionizing (low LET) radiation, at low doses the survival curve starts out straight on the log-linear plot with a finite initial slope, at higher doses, the curve bends. At very high doses, the survival curve tends to be straightened again, and the surviving fraction returns to an exponential function of dose. By contrast, for densely ionizing(high LET) radiations, the survival curve is a straight line from the origin, survival approximates to an exponential function of dose.
3.4 The target localization and the biological outcomesTo take SRT as an example, we mean treatments that are based on 3-D anatomic information and use treatment fields that conform as closely as possible to the target volume in order to deliver adequate dose to the tumor and minimum possible dose to normal tissue. The concept of conformal dose distribution has also been extended to include clinical objectives such as maximizing TCP and minimizing NTCP. Thus, this technique encompasses both the physical and biologic rationales in achieving the desired clinical results.
Obstacles to achieving optimal dose distribution lie in the knowledge of the tumor extent. Despite the modern advances in imaging, the clinical target volume (CTV) is often not fully discernible. Depending on the invasive capacity of diseases, what is imaged is usually not the CTV but what is called the gross tumor volume (GTV). Thus, if CTV drawn on the cross-sectional images do not fully include the microscopic spread of the disease, the SRT loses its meaning of being conformal. If any part of the cancer tissue is missed or seriously underdosed, it will inevitably result in failure despite all the care and effort expended in treatment planning, treatment delivery, and quality assurance. From the TCP point of view, accuracy in localization of CTV is more critical in SRT than in techniques that use generously wide fields and simpler beam arrangements to compensate for the uncertainty in tumor localization.
In addition to the difficulties in the assessment and localization of CTV, there are other potential errors that must be considered. Patient motion, including that of tumor volume, critical organs, and external fiducially marks during imaging, simulation, and treatment, can give rise to systematic and random errors that must be accounted for when designing the planning target volume (PTV). If sufficient margins have been allowed for in the localization of PTV, the beam apertures are then shaped to conform and adequately cover the PTV (e.g., within 95% to 105% isodose surface relative to prescribed dose). In the design of conformal fields to adequately treat the PTV, consideration must be given to the cross-beam profile, penumbra, and lateral radiation transport as a function of depth, off-axis distance, and tissue density. Therefore, sufficient margins must be given between the PTV outline and the field boundary to ensure adequate dose to the PTV at every treatment session.
4 The interactions between cancer and microenvironment determine the final outcomes of RT 4.1 Local target effectsMammalian cells exposed to ionizing radiation can die through various mechanisms: mitotic-linked cell death, necrotic cell death, apoptotic cell death, autophagic cell death and bystander-induced cell death[22-27]. Regarding normal tissues that are exposed to ionizing radiation, mitotic-linked cell death and apoptotic death have been studied the most. Programmed cell death, including apoptotic cell death, autophagic cell death, mitotic catastrophe, anoikis, excitotoxicity, Wallerian degeneration, pyronecrosis, entosis, paraptosis, etc[28-29], is characterized by the cascade regulation by genes and the integrity of cell membranes is maintained even after cell death. For such non-programmed cell death as necrosis, the breakage of membranes will lead to the leakage of cytosolic ingredients, the local inflammation occurs. The following events might happen locally after radiation: cancer cells fall on death with or without the leakage of contents, the release of cancer antigens, the microenvironment damage including vessel damage, deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1, enhancement of platelet adhesion to the extracellular matrix [30-31]. Simultaneously, radiation could stimulate secretion of pro-inflammatory cytokines and induce bystander effect[32-34]. The knowledge of different types of cancer cell death and the interaction between cancer cells and microenvironment limits the optimization of RT.
4.2 Abscopal non-target EffectsIn 1953, Mole[35] found that irradiation of local tissues could induce biological effects in the same or different tissues far from the irradiation field, and presented the concept of abscopal. In clinical treatment of metastatic tumors irradiated locally, the significant reduction of non-irradiation tumor volume occurs, which is known as abscopal effect[36]. Here the abscopal effects refer to the same or opposite side, or the distant effects, we also name them as non-target effects.
The abscopal effect is closely related to the immune system. Radiotherapy could be an approach to activate the systemic immune responses which play important roles in the development of malignant tumors. The local radiotherapy could directly kill tumor cells, also promote the release of pro-inflammatory cytokines by immune cells and act on non-irradiated tumor sites, which induce chromosome breakage or distortion in non-irradiated tumor cells[37]. It could also change the biological phenotype of the non-killed tumor cells, enhance the sensitivity to the immune system, and lead to highly anti-tumor immunological effect[38]. Local radiotherapy could enhance the immune responses to distant metastasis[39], also cause damage to normal tissues in non-irradiated fields, such as radiation-induced lung injury, skeletal complications including local and systemic osteoporosis, osteonecrosis, fractures and non-malignant bone tumors, and abscopal metastasis[40-41].
4.3 Recurrence and metastasisAlthough the tumor has been treated with radiotherapy, the remaining part may have viable cancer cells which continue growth and develop into the same type of tumor in their original place after a period of time, i.e., recurrence happens. Metastasis is defined as malignant cancer cells detached from its primary site, and spread to other parts of the body through direct transmission, lymph, blood and other pathways, and even multiply in some organs.
Local recurrence and distant metastasis are the main causes of treatment failure and patient death of malignant tumors, cancer stem cell(CSC) is one of the most important factors[42]. In 2006, American Association for Cancer Research (AACR) defined CSC as a cell within a tumor that possess the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor[43]. CSC is similar to normal stem cells, with main features of unlimited self-renewal ability and differentiation potential, high telomerase activity, high tumorigenic ability, and insensitivity to radiotherapy and chemotherapy[44]. Regimens without the consideration of CSC lead to misestimate the RT efficacy.
Epithelial-mesenchymal transition (EMT), the process of epithelial cells transforming into mesenchymal cells and obtaining the ability of invasion and migration, plays pivotal roles in the differentiation of human embryonic stem cells. EMT could make tumor cells obtain self-renewal capacity and stem cell characteristics, promote the production of CSC and tumor invasion and metastasis[45-46]. It also involved multiple signal transduction pathways and complex molecular mechanisms[47]. The occurrence of EMT and the regulation of CSC provides a new perspective for tumor research and the treatment of EMT and CSC is expected to reduce the recurrence and metastasis of tumors.
5 Radiation-induced second primary cancers (RISPC)The radiation-induced second primary cancers (RISPC) is one the most serious long-term consequences of cancer treatment. In the era of modern radiation therapy, the compromise between the reductions in deterministic radiation-induced toxicities through highly conformal devices may be impacting the stochastic risk of second malignancies. Studies have documented that the risk of RISPC is increasing among patients received radiation therapy as part of their primary anticancer treatment.
Although this increase in risk of RISPC may be small[48-49] in absolute terms, Murray et al[50] showed the risks of RISPC following prostate radiotherapy using 3D-CRT, and IMRT, volumetric modulated arc therapy (VMAT), flattening filter free (FFF) and stereotactic ablative radiotherapy (SABR). SABR resulted in lower RISPC risks at all sites relative to 3D-CRT, FFF resulted in lower RISPC risks in out-of-field tissues relative to equivalent flattened techniques, with increasing impact in organs at greater distances from the field. Relative to 10 MV 3D-CRT, 6 MV IMRT or VMAT with flattening filter increased risk of RISPC in out-of-field organs by up to 26% and 55%, respectively.Acalculated relative RISPC risk benefit from SABR and FFF techniques was theoretically predicted, although absolute RISPC risks were low for all techniques. Hall and Wuu[51] thought IMRT was likely to double the incidence of RISPC compared with conventional radiotherapy from about 1% to 1.75% for patients surviving 10 years. While Chargari et al[52] and Murray[53] thought clinical studies with short follow-up have not corroborated the hypothesis that more modern techniques such as IMRT and brachytherapy would drastically increase RISPC. Take prostate cancer as an example, the risk of RISPC appears in the range of 1 in 220 to 290 over all durations of follow-up[53]. Chargari et al[52] thought the risk inherent to these technologies remained uncertain and closely depended on the chosen risk model. According to the linear no-threshold model, the risk of RISPC could be twice higher with IMRT, as compared to conformal radiation therapy. It seems that only proton therapy could decrease both high and low doses delivered to non-target volumes[50]. Clinical follow-up remains insufficient and even the lower doses (< 5 Gy) of radiation could not be neglected, for IMRT or SRT significantly increase tissue volumes receiving low doses.
Although the underlying mechanism of the RISPC is uncertain, the following factors might affect the occurrence of RISPC: ① Irradiation volume: both the increased exposure dose of the whole body and the time extension of tissues exposure could increase the occurrence of RISPC[51]. ② Age factor: the age might be greatly associated with the occurrence of RISPC, and the risk is higher in younger patients compared with those older[54-55]. ③ Radiation dose: the possibility of medium dose (30-60 Gy) induced RISPC is higher than large dose (above 60 Gy)[56]. It′s probably because the tumor and normal cells were more killed when exposed to large dose of radiation, and the less cells were survived by sublethal damage.
To sum up, the above-mentioned theories are facing challenge and limiting the progress of modern radiotherapy, which needs further study by radiobiologists and the revelation of these theories will not only broaden and deepen the radiobiological basis, but also contribute to the practice in the clinic and improve the treatment efficacy of modern radiotherapy.
Conflict of interest statement The authors declare no conflicts of interest with regard to this manuscriptContribution statement of authors Ma Shumei, design and writing as a whole; Liang Zhenzhen, writing of part 1 and 2; Chen Qiao and Guo Yutian, writing of part 3 and 4; Liu Rui, the editing and typesetting; Liu Xiaodong, the advising and modifying of this manuscript
| [1] | Roentgen WC. On a new kind of ray (the first report)[J]. Munch Med Wochenschr, 1959, 101 : 1237-1239. |
| [2] | Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer[J]. J Clin Oncol, 2014, 32 (26): 2913-2919. DOI:10.1200/JCO.2014.55.1143. |
| [3] | Mould RF. A century of X-rays and radioactivity in medicine[M]. Bristol: IOPP, 1993: 126-147. |
| [4] | Brahme A. Development of radiation therapy optimization[J]. Acta Oncol, 2000, 39 (5): 579-595. DOI:10.1080/028418600750013267. |
| [5] | Leksell L. The stereotaxic method and radiosurgery of the brain[J]. Acta Chir Scand, 1951, 102 (4): 316-319. |
| [6] | Cong Y, Wu SK. Stereotactic radiation therapy in the era of precision medicine for cancer[J]. Clin J Appl Physiol, 2015, 31 (6): 491-497. |
| [7] | Teh B. Image-guided stereotactic body radiation therapy (SBRT):an emerging treatment paradigm with a new promise in radiation oncology[J]. Biomed Imaging Interv J, 2007, 3 (1): e5 DOI:10.2349/biij.3.1.e5. |
| [8] | Ceniceros L, Aristu J, Castañón E, et al. Stereotactic body radiotherapy (SBRT) for the treatment of inoperable stage I non-small cell lung cancer patients[J]. Clin Transl Oncol, 2016, 18 (3): 259-268. DOI:10.1007/s12094-015-1361-4. |
| [9] | Rombouts SJ, Vogel JA, van Santvoort HC, et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer[J]. Br J Surg, 2015, 102 (3): 182-193. DOI:10.1002/bjs.9716. |
| [10] | Lischalk JW, Kaplan ID, Collins SP. Stereotactic body radiation therapy for localized prostate cancer[J]. Cancer J, 2016, 22 (4): 307-313. DOI:10.1097/PPO.0000000000000209. |
| [11] | Meng M, Wang H, Zeng X, et al. Stereotactic body radiation therapy:A novel treatment modality for inoperable hepatocellular carcinoma[J]. Drug Discov Ther, 2015, 9 (5): 372-379. DOI:10.5582/ddt.2015.01056. |
| [12] | Strom T, Wishka C, Caudell JJ. Stereotactic body radiotherapy for recurrent unresectable head and neck cancers[J]. Cancer Control, 2016, 23 (1): 6-11. DOI:10.1177/107327481602300103. |
| [13] | Amini A, Yeh N, Gaspar LE, et al. Stereotactic body radiation therapy (SBRT) for lung cancer patients previously treated with conventional radiotherapy:a review[J]. Radiat Oncol, 2014, 9 : 210 DOI:10.1186/1748-717X-9-210. |
| [14] | Hall EJ, Giaccia AJ. Chapter 23: time, dose, and fractionation in radiotherapy//Hall EJ, Giaccia AJ. Radiobiology for the radiologist[M].7th ed. Philadelphia: LWW, 2012: 391-411. |
| [15] | Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction[J]. Semin Radiat Oncol, 2008, 18 (4): 234-239. DOI:10.1016/j.semradonc.2008.04.004. |
| [16] | Shibamoto Y, Miyakawa A, Otsuka S, et al. Radiobiology of hypofractionated stereotactic radiotherapy:what are the optimal fractionation schedules?[J]. J Radiat Res, 2016, 57 (Suppl 1): i76-i82. DOI:10.1093/jrr/rrw015. |
| [17] | Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose:useful tools in understanding potency of ablative radiotherapy[J]. Int J Radiat Oncol Biol Phys, 2008, 70 (3): 847-852. DOI:10.1016/j.ijrobp.2007.10.059. |
| [18] | Guerrero M, Carlone M. Mechanistic formulation of a lineal-quadratic-linear (LQL) model:split-dose experiments and exponentially decaying sources[J]. Med Phys, 2010, 37 (8): 4173-4181. DOI:10.1118/1.3456927. |
| [19] | Wang JZ, Huang Z, Lo SS, et al. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy[J]. Sci Transl Med, 2010, 2 (39): 39ra48 DOI:10.1126/scitranslmed.3000864. |
| [20] | Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery[J]. Semin Radiat Oncol, 2008, 18 (4): 240-243. DOI:10.1016/j.semradonc.2008.04.005. |
| [21] | Hall EJ. Radiation dose-rate:a factor of importance in radiobiology and radiotherapy[J]. Br J Radiol, 1972, 45 (530): 81-97. DOI:10.1259/0007-1285-45-530-81. |
| [22] | Zhong R, Xin R, Chen Z, et al. The role of deoxycytidine kinase (dCK) in radiation-induced cell death[J]. Int J Mol Sci, 2016, 17 (11): 1939 DOI:10.3390/ijms17111939. |
| [23] | Cui L, Song Z, Liang B, et al. Radiation induces autophagic cell death via the p53/DRAM signaling pathway in breast cancer cells[J]. Oncol Rep, 2016, 35 (6): 3639-3647. DOI:10.3892/or.2016.4752. |
| [24] | Wang B, Chen Z, Yu F, et al. Hsp90 regulates autophagy and plays a role in cancer therapy[J]. Tumour Biol, 2016, 37 (1): 1-6. DOI:10.1007/s13277-015-4142-3. |
| [25] | Yu F, Chen Z, Wang B, et al. The role of lysosome in cell death regulation[J]. Tumour Biol, 2016, 37 (2): 1427-1436. DOI:10.1007/s13277-015-4516-6. |
| [26] | Liang N, Zhong R, Hou X, et al. Ataxia-telangiectasia mutated (ATM) participates in the regulation of ionizing radiation-induced cell death via MAPK14 in lung cancer H1299 cells[J]. Cell Prolif, 2015, 48 (5): 561-572. DOI:10.1111/cpr.12203. |
| [27] | Liang N, Jia L, Liu Y, et al. ATM pathway is essential for ionizing radiation-induced autophagy[J]. Cell Signal, 2013, 25 (12): 2530-2539. DOI:10.1016/j.cellsig.2013.08.010. |
| [28] | Kroemer G, Galluzzi L, Vandenabeele P, et al. Classification of cell death:recommendations of the Nomenclature Committee on Cell Death 2009[J]. Cell Death Differ, 2009, 16 (1): 3-11. DOI:10.1038/cdd.2008.150. |
| [29] | Galluzzi L, Vitale I, Abrams JM, et al. Molecular definitions of cell death subroutines:recommendations of the Nomenclature Committee on Cell Death 2012[J]. Cell Death Differ, 2012, 19 (1): 107-120. DOI:10.1038/cdd.2011.96. |
| [30] | Wang J, Zheng H, Ou X, et al. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine:possible link between endothelial dysfunction and chronic radiation fibrosis[J]. Am J Pathol, 2002, 160 (6): 2063-2072. DOI:10.1016/S0002-9440(10)61156-X. |
| [31] | Verheij M, Dewit LG, Boomgaard MN, et al. Ionizing radiation enhances platelet adhesion to the extracellular matrix of human endothelial cells by an increase in the release of von Willebrand factor[J]. Radiat Res, 1994, 137 (2): 202-207. DOI:10.2307/3578813. |
| [32] | Shan YX, Jin SZ, Liu XD, et al. Ionizing radiation stimulates secretion of pro-inflammatory cytokines:dose-response relationship, mechanisms and implications[J]. Radiat Environ Biophys, 2007, 46 (1): 21-29. DOI:10.1007/s00411-006-0076-x. |
| [33] | Liu SZ, Jin SZ, Liu XD. Radiation-induced bystander effect in immune response[J]. Biomed Environ Sci, 2004, 17 (1): 40-46. |
| [34] | Liu XD, Ma SM, Liu SZ. Effects of 0.075 Gy x-ray irradiation on the expression of IL-10 and IL-12 in mice[J]. Phys Med Biol, 2003, 48 (13): 2041-2049. DOI:10.1088/0031-9155/48/13/315. |
| [35] | Mole RH. Whole body irradiation; radiobiology or medicine?[J]. Br J Radiol, 1953, 26 (305): 234-241. DOI:10.1259/0007-1285-26-305-234. |
| [36] | Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy:a clinical review for the radiobiologist[J]. Cancer Lett, 2015, 356 (1): 82-90. DOI:10.1016/j.canlet.2013.09.018. |
| [37] | Lorimore SA, Chrystal JA, Robinson JI, et al. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation[J]. Cancer Res, 2008, 68 (19): 8122-8126. DOI:10.1158/0008-5472.CAN-08-0698. |
| [38] | Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy:using immunotherapy to make a rare event clinically relevant[J]. Cancer Treat Rev, 2015, 41 (6): 503-510. DOI:10.1016/j.ctrv.2015.03.011. |
| [39] | Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers[J]. Int J Mol Sci, 2014, 15 (1): 927-943. DOI:10.3390/ijms15010927. |
| [40] | Ishiyama H, Teh BS, Ren H, et al. Spontaneous regression of thoracic metastases while progression of brain metastases after stereotactic radiosurgery and stereotactic body radiotherapy for metastatic renal cell carcinoma:abscopal effect prevented by the blood-brain barrier?[J]. Clin Genitourin Cancer, 2012, 10 (3): 196-198. DOI:10.1016/j.clgc.2012.01.004. |
| [41] | Kwon JW, Huh SJ, Yoon YC, et al. Pelvic bone complications after radiation therapy of uterine cervical cancer:evaluation with MRI[J]. AJR Am J Roentgenol, 2008, 191 (4): 987-994. DOI:10.2214/AJR.07.3634. |
| [42] | Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer[J]. Cell Stem Cell, 2007, 1 (3): 313-323. DOI:10.1016/j.stem.2007.06.002. |
| [43] | Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells-perspectives on current status and future directions:AACR Workshop on cancer stem cells[J]. Cancer Res, 2006, 66 (19): 9339-9344. DOI:10.1158/0008-5472.CAN-06-3126. |
| [44] | Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance[J]. Nat Rev Cancer, 2008, 8 (7): 545-554. DOI:10.1038/nrc2419. |
| [45] | Kalluri R. EMT:when epithelial cells decide to become mesenchymal-like cells[J]. J Clin Invest, 2009, 119 (6): 1417-1419. DOI:10.1172/JCI39675. |
| [46] | Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease[J]. Cell, 2009, 139 (5): 871-890. DOI:10.1016/j.cell.2009.11.007. |
| [47] | Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells[J]. Cell, 2008, 133 (4): 704-715. DOI:10.1016/j.cell.2008.03.027. |
| [48] | Murray L, Henry A, Hoskin P, et al. Second primary cancers after radiation for prostate cancer:a review of data from planning studies[J]. Radiat Oncol, 2013, 8 : 172 DOI:10.1186/1748-717X-8-172. |
| [49] | Chargari C, Cosset JM. The issue of low doses in radiation therapy and impact on radiation-induced secondary malignancies[J]. Bull Cancer, 2013, 100 (12): 1333-1342. DOI:10.1684/bdc.2013.1855. |
| [50] | Murray LJ, Thompson CM, Lilley J, et al. Radiation-induced second primary cancer risks from modern external beam radiotherapy for early prostate cancer:impact of stereotactic ablative radiotherapy (SABR), volumetric modulated arc therapy (VMAT) and flattening filter free (FFF) radiotherapy[J]. Phys Med Biol, 2015, 60 (3): 1237-1257. DOI:10.1088/0031-9155/60/3/1237. |
| [51] | Hall EJ, Wuu CS. Radiation-induced second cancers:the impact of 3D-CRT and IMRT[J]. Int J Radiat Oncol Biol Phys, 2003, 56 (1): 83-88. DOI:10.1016/S0360-3016(03)00073-7. |
| [52] | Chargari C, Goodman KA, Diallo I, et al. Risk of second cancers in the era of modern radiation therapy:does the risk/benefit analysis overcome theoretical models?[J]. Cancer Metastasis Rev, 2016, 35 (2): 277-288. DOI:10.1007/s10555-016-9616-2. |
| [53] | Murray L, Henry A, Hoskin P, et al. Second primary cancers after radiation for prostate cancer:a systematic review of the clinical data and impact of treatment technique[J]. Radiother Oncol, 2014, 110 (2): 213-228. DOI:10.1016/j.radonc.2013.12.012. |
| [54] | Lönn S, Gilbert ES, Ron E, et al. Comparison of second cancer risks from brachytherapy and external beam therapy after uterine corpus cancer[J]. Cancer Epidemiol Biomarkers Prev, 2010, 19 (2): 464-474. DOI:10.1158/1055-9965.EPI-09-0892. |
| [55] | Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers[J]. Int J Radiat Oncol Biol Phys, 2006, 65 (1): 1-7. DOI:10.1016/j.ijrobp.2006.01.027. |
| [56] | Seydel HG. The risk of tumor induction in man following medical irradiation for malignant neoplasm[J]. Cancer, 1975, 35 (6): 1641-1645. DOI:10.1002/1097-0142(197506)35:6<1641::AID-CNCR2820350625>3.0.CO;2-J. |
 2019, Vol. 39
2019, Vol. 39


