2. 武汉大学人民医院肿瘤科 430060;
3. 湖北省肿瘤医院放疗科, 武汉 430079
2. Department of Oncology, Renmin Hospital of Wuhan University, Wuhan 430060, China;
3. Department of Radiation Oncology, Hubei Cancer Hospital, Wuhan 430079, China
乳腺癌是女性最常见的恶性肿瘤之一,根治术后进行术后放疗是局部中晚期乳腺癌综合治疗的重要治疗方式,术后辅助放疗可明显降低复发概率,提高患者生存率[1-3]。目前根治术后放疗方法主要分为:三维适形放疗(three dimensional conformal radiotherapy, 3D-CRT)、调强放疗(intensity modulated radiation therapy, IMRT)、容积旋转调强(volumetric-modulated arc therapy,VMAT)、3D-CRT和IMRT组合放疗,以及X射线和电子线结合治疗等[4-8]。乳腺癌根治术后放疗靶区(胸壁、锁骨上下区、内乳区及部分腋窝高危区)范围广,且形状不规则,整体呈凹形,个体差异性较大。对于左侧乳腺癌根治术后患者,危及器官(organs at risk, OARs)肺、心脏等与靶区毗邻,放疗计划设计难度较高,其随着靶区弯曲幅度增大而增大。IMRT的剂量适形、均匀性等优势已在诸多研究中得以体现[4-5, 8],但在计划设计时,都是基于靶区作为一个整体,少有对靶区进行分割后给予相应的目标计划方案的IMRT计划研究报告。本研究选择左侧局部晚期乳腺癌根治术后患者,基于动态IMRT技术,设计靶区分割式放射治疗计划(target segmented plan, TSP)、与TSP优化参数相同但未分割靶区计划(Non-TSP)和常规8野调强计划(8F-IMRT),分析比较这3种治疗计划方式对靶区和OARs剂量学的差异,同时尝试探寻靶区弯曲幅度变化对计划的影响,为乳腺癌根治术后患者的放疗计划设计方案优化提供新的思路。
材料与方法1.入组病例:选取从2017年6月至2018年11月在武汉大学人民医院放疗科收治的30例左侧局部晚期乳腺癌根治术后患者资料,所有患者年龄34~68岁(中位年龄53岁),术后病理分期为:pT3~4,腋窝淋巴结转移数目>3个;所选患者靶区弯曲幅度较大,入组标准为CT横断面显示胸壁靶区两侧后界连线切肺最大深度>2 cm。将所有患者按照连线切肺最大深度,分为<3 cm组、3~4 cm组和>4 cm组,探寻切肺深度的变化进而带来计划的靶区及危及器官剂量变化的趋势。
2.模拟定位及靶区勾画:所有患者采用仰卧位,固定方式采用碳纤维定位体架和真空垫,双手过头握同侧固定杆,头偏向健侧。采用CT(GE-HISPEED,美国GE公司)进行肺部扫描,扫描参数:螺距1.75:1,速度35 mm/层,层厚5 mm,上界至环状软骨,下界至上腹部,因胸壁表面需要剂量照射,在胸壁CT扫描时加1 cm等效组织补偿膜(专用记号笔标定补偿膜放置位置)。定位CT数据传至Varian Eclipse计划系统(美国Varian公司),选定加速器为Varian 23IX,6 MV X射线,射野最大为40 cm×40 cm,多叶准直器(MLC)在20 cm×20 cm射野范围内,等中心处为5 mm,超出范围为1 cm。由同一名资深放疗专业医师根据放射治疗协作组织(RTOG)乳腺癌勾画指南[9]勾画临床靶区(CTV)和肺、心脏、脊髓、健侧乳腺和肱骨头等危及器官,并对其给予处方剂量和危及器官限制剂量。结合本单位整个治疗流程中的系统误差和摆位随机误差以及患者呼吸运动[10],将临床靶区(CTV)外放0.7 cm生成计划靶区体积(PTV);其次因MV级X射线特有的建成区内剂量的不准确性[11],把PTV距离皮肤外轮廓(包含补偿膜)往内收3 mm。
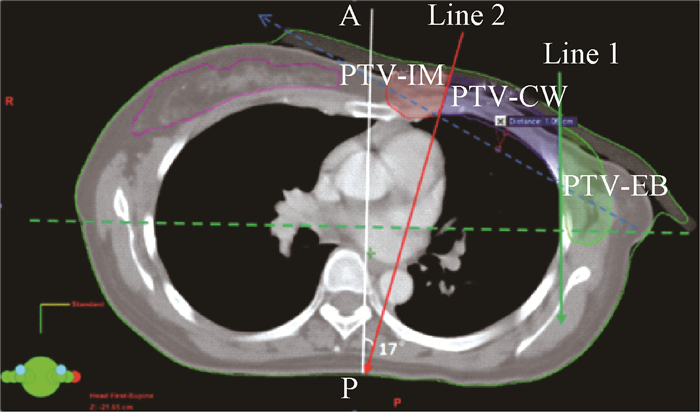
3.TSP的靶区分割方式:将PTV分为4个分区:锁骨上区(PTV-supraclavicular,PTV-SC)、内乳区(PTV-internal mammary,PTV-IM)、胸壁区(PTV-chest wall,PTV-CW)和外侧区(PTV-external breast,PTV-EB)。PTV-SC与RTOG定义一致,其他3个区主要由和两条线来分割,Line1是每层横截面中两肺最大间距连线的垂线;Line2是穿过内乳血管向外侧7~10 mm,且偏A~P方向约20°的直线,这两条线将原PTV(除去PTV-SC)分为PTV-IM、PTV-CW、PTV-EB3个区,见图 1。建议两条线与胸壁的交点的连线与健侧乳腺无交叉(蓝色虚线),且与患侧肺最外侧的最大距离 < 2 cm。
|
注:蓝色.胸壁区;红色.内乳区;绿色.外侧区 图 1 左侧局部晚期乳腺癌根治术后患者靶区分割 Figure 1 Schematic diagram of target segmentation in left-sided breast cancer patients with postmastectomy |
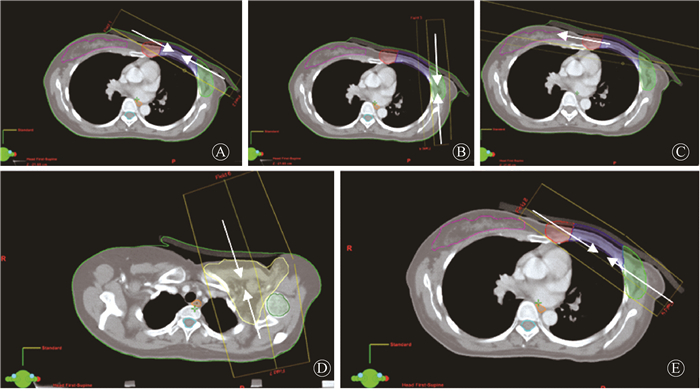
4. TSP和Non-TSP计划设计:TSP设置9个射野,射野等中心设置为PTV几何等中心,PTV-SC设计2野,射野角度避开脊髓和食管,两野呈射野内侧重合(半野照射);PTV-CW设计2野,切线野对穿,两野呈射野内侧重合(半野照射);PTV-IM设计1野,角度约为100°,避开心脏;PTV-EB同理给予2野呈半野对穿野,角度设置标准为尽量避开旁边肺组织,再在PTV切线方向加2野(主要用于剂量衔接、减小剂量热点和冷点概率和增加靶区剂量均匀性)。射野设置见图 2。各射野均采取JAW-Fixed模式,JAW的位置相对于靶区的具体设置为:各个分割靶区在射野方向观(BEV)上,前方向外放2 cm;后方向外放0.1 cm且保持半野照射;头、脚方向外放1 cm,左、右方向外放0.5 cm。Non-TSP计划射野数量、角度以及优化权重参数都与TSP一样,但不采取靶区分割(射野的二级准直器JAW未采用Fixed模式),Non-TSP的JAW范围都以整个PTV为目标。Non-TSP的作用是TSP的对照计划,主要验证靶区分割是否有效,二是与常规IMRT射野方向设置不同,间接验证射野方向对计划的影响。
|
注:A.胸壁区;B.外侧区; C.内乳区;D.锁骨上区;E.计划靶区体积 图 2 左侧局部晚期乳腺癌根治术后患者靶区分割式放射治疗计划9野示意图 Figure 2 Schematic diagram of 9 fields setting of TSP in left-sided breast cancer patients with postmastectomy |
5.常规8F-IMRT计划设计:乳腺根治术后计划射野方向选择主要为切线方向,本组总照射野数为8野,与TSP射野数量仅相差一野。以减小射野数量带来的可能影响。主野为两夹角呈射野内侧重合的半野照射,靠健侧乳腺切线野为界,向上间隔10°设置3野,靠乳腺外侧区切线野向上间隔10°设置3野。
6.计划优化条件以及危及器官限量:计划目标剂量体积直方图(DVH)为V100%达到95%体积(本组V50 Gy=95%)为最低处方要求。每个患者的3个计划目标优化参数和权重相同。危机器官限量,患侧肺Dmean ≤ 15 Gy, V5 Gy≤ 60%, V10 Gy≤ 40%, V20 Gy≤ 28%, V30 Gy≤ 20%;健侧乳腺Dmax≤ 40 Gy; Dmean≤5 Gy;脊髓Dmax ≤ 45 Gy; 肱骨头Dmean≤ 40 Gy。心脏Dmean ≤ 10 Gy, V20 Gy≤ 30%, V30 Gy≤ 15% [12]。
7. 3种计划方式靶区和OAR剂量学参数比较:靶区评价主要采用均匀性指数(homogeneity index, HI)和适形指数(conformity index, CI),其中HI=(D2%-D98%)/Dp×100%,D2%、D98%分别为2%、98%靶区体积对应剂量,Dp为靶区给予处方剂量;CI=VT.ref/VT×VT.ref /Vref,VT.ref为靶区接受处方剂量体积,VT为靶区体积,Vref为处方剂量包含总体积。同时,PTV比较参数为D2%、D98%、Dmean。OAR比较参数为肺的V5 Gy、V10 Gy、V20 Gy、Dmean;健侧乳腺的Dmean;心脏的V5 Gy、V10 Gy、V20 Gy、Dmean;脊髓Dmax;肱骨头Dmean。
8.评估靶区切肺深度变化对计划的影响:对于一个从DVH图读取的剂量参数x,若要认为TSP计划随着深度增加好于Non-TSP和8F-IMRT,则需要满足下列关系:x:D(N-T, < 3 cm)<D(N-T, 3~4 cm)<D(N-T, >4 cm)和x:D(8F-T, < 3 cm)<D(8F-T, 3~4 cm)<D(8F-T, >4 cm),这里D(N-T, < 3 cm)为 < 3 cm组x的Non-TSP与TSP的差值;D(8F-T, < 3 cm)为 < 3 cm组x的8F-IMRT与TSP的差值,其他类推。
9.统计学处理:采用SPSS 25.0软件进行统计分析,以x±s表示,数据非正态分布,3组之间的比较采用非参数Friedman检验, P<0.05的参数再进行两两计划的比较,选择非参数Wilcoxon带符秩检验。P<0.05为差异有统计学意义。
结果1.PTVs剂量参数对比:TSP、Non-TSP和8F-IMRT计划都能满足靶区剂量分布要求,即超过95%体积靶区获得50 Gy。TSP的D98%低于Non-TSP和8F-IMRT差异有统计学意义(Z=-3.294、-3.266,P<0.05);3种计划的HI和CI差异无统计学意义(P>0.05);TSP的Dmean高于8F-IMRT(Z=-3.181,P<0.05);Non-TSP的加速器治疗的跳数(monitor unit,MU)高于TSP和8F-IMRT(Z=-3.04、-2.669,P<0.05), 见表 1。
|
|
表 1 左侧局部晚期乳腺癌根治术后患者不同治疗计划计划靶区体积剂量参数(x±s) Table 1 PTV dose parameters of three plans in left-sided breast cancer patients with postmastectomy(x±s) |
2.患侧肺及危及器官剂量比较:V5 G、V10 Gy、V20 Gy和Dmean,TSP都明显低于Non-TSP和8F-IMRT计划(V5 Gy:Z=-3.408、-3.408;V10 Gy:Z=-3.408、-3.408;V20 Gy:Z=-3.408、-3.124;Dmean:Z=-3.408、-3.408,P<0.05)。TSP的心脏低剂量受照体积明显低于Non-TSP和8F-IMRT,对应V5 Gy、V10 Gy和Dmean差异均具有统计学意义(V5 Gy:Z=-3.408、-3.408;V10 Gy:Z=-3.408、-3.408;Dmean:Z=-3.408、-3.408,P<0.05)。Non-TSP健侧乳腺的Dmean高于TSP和8F-IMRT(Z=-2.954、-2.215,P<0.05);健侧乳腺和脊髓的Dmax无统计学意义(P>0.05);8F-IMRT肱骨头Dmean高于TSP和Non-TSP(Z=-3.01、-2.442,P<0.05),见表 2。
|
|
表 2 左侧局部晚期乳腺癌根治术后患者不同治疗计划危及器官剂量参数(x±s) Table 2 OARs′dose parameters of three plans in left-sided breast cancer patients with postmastectomy(x±s) |
3.患者靶区后界连线切肺最大深度对计划的影响:患者靶区后界连线切肺最大深度<3 cm 18例,3~4 cm 8例,>4 cm 4例。随着靶区切肺深度的增加,患侧肺和心脏的目标参数均值变化幅度值均满足D(N-T, <3 cm)<D(N-T, 3~4 cm)<D(N-T, >4 cm)和D(8F-T, <3 cm)<D(8F-T, 3~4 cm)<D(8F-T, >4 cm),幅度差值在低剂量区域更为明显,其目标参数幅度差均值统计见表 3。
|
|
表 3 左侧局部晚期乳腺癌根治术后患者不同切肺深度计划间均值差值肺和心脏的受照体积变化 Table 3 Changes in the exposure volume of lung and heart between the mean values of different plans indifferent depth of incision into the lung |
讨论
局部晚期乳腺癌根治术后的放射治疗计划的选择呈现多样性,选择的依据在于勾画的靶区与心脏、肺等OARs的相对位置。靶区剂量覆盖和OARs一定体积受到剂量照射或最高剂量限值可能引起相应的并发症是决定治疗计划是否可行的重要因素。在乳腺保乳术后患者,切线野(或切线适形射野+调强组合(80% :20%剂量分配)计划能有效降低患侧肺的V5 Gy、V10 Gy,在临床计划中得到广泛运用[13-14],但是对于靶区弯曲幅度较大乳腺根治术后患者,切线野(包括野中野)就会让患侧肺V20 Gy过大且靶区适形度较差而不宜采用[4, 15],因此,本研究选择单纯IMRT技术,尤其当靶区包括内乳淋巴结区时,随着过腋中线距离的增大,整个靶区凹形幅度也增大,对于计划设计的难度也大幅提高。HI、CI可以客观地衡量放疗剂量分布体积与靶区体积的大小和形状的适形性。在本研究中,TSP的CI和HI在TSP、Non-TSP和8F-IMRT这3种计划设计方法中无明显差异。3种计划方式在达了靶区处方剂量要求的同时,靶区内剂量的均匀性达到了治疗计划的要求。本研究中所有计划的CI均值位于0.63~0.65,整体值不高,主要原因是入组病例靶区弯曲幅度较大的缘故,在适形度和危及器官的限量之间,优先考虑OAR的安全。
放射性肺炎风险(pneumonitis risk, PR)是胸部放疗患者常见的重要放疗并发症,常规的剂量学影响因子包括V5 Gy、V10 Gy和V20 Gy等,但究竟哪种为主要的影响因子尚存争议。Blom等[16]和Gopal等[17]认为患侧肺V20 Gy与PR的发生呈正相关性;Willner等[18]则认为PR发生率在V10 Gy每增加10%也呈10%的幅度增长;Yorke等[19]认为肺V5 Gy和V10 Gy可能是放射性肺损伤的有效预测因子。本研究发现TSP计划的患侧肺整体受量都低于Non-TSP和8F-IMRT,低剂量区(V5 Gy和V10 Gy)和平均剂量(Dmean)降幅明显。由此可见,TSP计划可以在保证靶区受到足量照射的同时可以明显降低患侧肺部的照射剂量和体积,这样可能减少放射性肺损伤的发生。乳腺癌放射治疗会对健侧乳腺的产生一定的影响。Popescu等[20]认为在RapidArc治疗模式中健侧乳腺Dmean < 3.2 Gy,可明显减小由放射治疗带来的二次致癌风险,尤其对于年轻的女性患者。本研究中3种计划方式中健侧乳腺Dmean均>3.2 Gy,这主要是由于放疗靶区包括了内乳淋巴结,患者靶区勾画弯曲幅度较大。即使在这种情况下,TSP中健侧乳腺Dmean仍低于Non-TSP和8F-IMRT,但3种计划绝对差值较小,所以以健侧乳腺的受量来权衡计划的选择力较弱。Darby等[12]研究认为缺血性心脏病的发生于心脏受照射的Dmean呈线性关系,心脏平均受量每增加1 Gy,冠状动脉事件发生率可相对地提高7.4%。从本研究中3种计划方案的心脏平均剂量对比来看,相较于Non-TSP和8F-IMRT,TSP能降低心脏Dmean,这样可能减少心脏损伤事件概率。
患者靶区后界连线切肺最大深度对计划的影响。随着靶区切肺深度的增加,患侧肺和心脏的目标参数均值变化幅度值均满足D (N-T, < 3 cm)<D (N-T, 3~4 cm)<D (N-T, >4 cm)和D (8F-T, < 3 cm)<D (8F-T, 3~4 cm)<D (8F-T, >4 cm),说明TSP计划随靶区切肺深度增加,其对患侧肺和心脏的保护更佳。对于患侧肺,在A组,D(N-T, A)在V20 Gy为0.57,D(8F-T, A)在V20 Gy为0.52,说明TSP在 < 3 cm对于患侧肺高剂量保护优势甚微;在V5 Gy和V10 Gy,3个切肺最大深度总体的均值变化幅度值为7.69~35.31,具有明显的优势;对于心脏,无论高剂量还是低剂量的保护,TSP的优势更为明显。
在乳腺癌根治术后的放射治疗中,可用的治疗技术较多。如Lai等[15]对比分析了3D-CRT与3种容积旋转调强(VMAT)计划(C-VMAT, M-VMAT和M-VMAT-F),从该研究结果来看,VMAT计划患侧肺的低剂量照射体积较大,V5 Gy最低已达到(70.3±5.8)%;Zhang等[21]对比分析了静态调强(step and shoot IMRT)与常规VMAT,最终发现VMAT在PTV和OAR参数都优于静态IMRT,这可能与静态IMRT的射野角度设置(300°、0°、40°、80°、110°)相关;Ma等[4]比较了静态IMRT(5fields-IMRT)、3D-CRT-field in field与2P-VMAT,3D-FIF的CI仅为0.27±0.07,适用性较差,5F-IMRT的V5 Gy、V10 Gy、V20 Gy全面优于2P-VMAT。Lai等[15]采用VMAT技术导致患侧肺的高剂量体积(V20 Gy)较低,但低剂量体积(V5 Gy)较高。综合看来VMAT对乳腺癌根治术后放射治疗的适用性尚存争议,可能依赖于各临床靶区形状及临床目的的不同。鉴于本文中所用计划系统无VMAT功能模块,本研究未将VMAT技术入组比较,与其他计划系统VMAT等其他技术的剂量比较是进一步研究方向。
本研究结果显示,Non-TSP计划射野方向与优化参数及权重都与TSP计划一致,但不对靶区进行分割加以限制,其患侧肺和心脏的剂量参数为3个计划中最差,且MU高于TSP和8F-IMRT,说明人为分割靶区的方法(TSP计划)的确会带来剂量学收益。
综上所述,对于靶区弯曲幅度较大的左侧局部晚期乳腺癌根治术后患者,TSP计划方式在确保放射治疗靶区受到足量处方剂量的同时,能有效的降低靶区周围OAR的受照剂量,尤其能明显降低患侧肺和心脏的V5 Gy和V10 Gy。TSP计划方式给剂量学带来的优势是否能转化为临床的获益还需要进一步研究证实。
利益冲突 无作者贡献声明 胡健负责论文的设计和撰写;李祥攀、阮长利和昌胜收集数据并进行统计分析;张爱华、戈伟和徐细明对论文的最终修订做出了贡献;韩光参与论文设计
| [1] |
Chung CS, Harris JR. Post-mastectomy radiation therapy:translating local benefits into improved survival[J]. Breast, 2007, 16(Suppl 2): S778-83. DOI:10.1016/j.breast.2007.07.018 |
| [2] |
Van de Steene J, Soete G, Storme G. Adjuvant radiotherapy for breast cancer significantly improves overall survival:the missing link[J]. Radiother Oncol, 2000, 55(3): 263-272. DOI:10.1016/s0167-8140(00)00204-8 |
| [3] |
Onitilo AA, Engel JM, Stankowski RV, et al. Survival comparisons for breast conserving surgery and mastectomy revisited:community experience and the role of radiation therapy[J]. Clin Med Res, 2015, 13(2): 65-73. DOI:10.3121/cmr.2014.1245 |
| [4] |
Ma C, Zhang W, Lu J, et al. Dosimetric comparison and evaluation of three radiotherapy techniques for use after modified radical mastectomy for locally advanced left-sided breast cancer[J]. Sci Rep, 2015, 5: 12274. DOI:10.1038/srep12274 |
| [5] |
Schubert LK, Gondi V, Sengbusch E, et al. Dosimetric comparison of left-sided whole breast irradiation with 3D-CRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy[J]. Radiother Oncol, 2011, 100(2): 241-246. DOI:10.1016/j.radonc.2011.01.004 |
| [6] |
Lin JF, Yeh DC, Yeh HL, et al. Dosimetric comparison of hybrid volumetric-modulated arc therapy, volumetric-modulated arc therapy, and intensity-modulated radiation therapy for left-sided early breast cancer[J]. Med Dosim, 2015, 40(3): 262-267. DOI:10.1016/j.meddos.2015.05.003 |
| [7] |
Zhang Q, Yu XL, Hu WG, et al. Dosimetric comparison for volumetric modulated arc therapy and intensity-modulated radiotherapy on the left-sided chest wall and internal mammary nodes irradiation in treating post-mastectomy breast cancer[J]. Radiol Oncol, 2015, 49(1): 91-98. DOI:10.2478/raon-2014-0033 |
| [8] |
Yang B, Wei XD, Zhao YT, et al. Dosimetric evaluation of integrated IMRT treatment of the chest wall and supraclavicular region for breast cancer after modified radical mastectomy[J]. Med Dosim, 2014, 39(2): 185-189. DOI:10.1016/j.meddos.2013.12.008 |
| [9] |
Gentile MS, Usman AA, Neuschler EI, et al. Contouring guidelines for the axillary lymph nodes for the delivery of radiation therapy in breast cancer:evaluation of the RTOG breast cancer atlas[J]. Int J Radiat Oncol Biol Phys, 2015, 93(2): 257-265. DOI:10.1016/j.ijrobp.2015.07.002 |
| [10] |
Qi XS, Hu A, Wang K, et al. Respiration induced heart motion and indications of gated delivery for left-sided breast irradiation[J]. Int J Radiat Oncol Biol Phys, 2012, 82(5): 1605-1611. DOI:10.1016/j.ijrobp.2011.01.042 |
| [11] |
Panettieri V, Barsoum P, Westermark M, et al. AAA and PBC calculation accuracy in the surface build-up region in tangential beam treatments. Phantom and breast case study with the Monte Carlo code PENELOPE[J]. Radiother Oncol, 2009, 93(1): 94-101. DOI:10.1016/j.radonc.2009.05.010 |
| [12] |
Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer[J]. N Engl J Med, 2013, 368(11): 987-998. DOI:10.1056/NEJMoa1209825 |
| [13] |
Caudell JJ, De Los Santos JF, Keene KS, et al. A dosimetric comparison of electronic compensation, conventional intensity modulated radiotherapy, and tomotherapy in patients with early-stage carcinoma of the left breast[J]. Int J Radiat Oncol Biol Phys, 2007, 68(5): 1505-1511. DOI:10.1016/j.ijrobp.2007.04.026 |
| [14] |
洪卫, 冉立, 卢冰, 等. 乳腺癌改良根治术后放疗降低患侧肺受量的剂量学研究[J]. 中华放射医学与防护杂志, 2011, 31(6): 684-687. Hong W, Ran L, Lu B, et al. Dosimetry of different techniques in postmastectomy radiation therapy on the ipsilateral lung[J]. Chin J Radiol Med Prot, 2011, 31(6): 684-687. DOI:10.3760/cma.j.issn.0254-5098.2011.06.016 |
| [15] |
Lai Y, Chen Y, Wu S, et al. Modified volumetric modulated arc therapy in left sided breast cancer after radical mastectomy with flattening filter free versus flattened beams[J]. Medicine (Baltimore), 2016, 95(14): e3295. DOI:10.1097/MD.0000000000003295 |
| [16] |
Blom GU, Wennberg B, Svane G, et al. Reduction of radiation pneumonitis by V20-constraints in breast cancer[J]. Radiat Oncol, 2010, 5: 99. DOI:10.1186/1748-717X-5-99 |
| [17] |
Gopal R, Tucker SL, Komaki R, et al. The relationship between local dose and loss of function for irradiated lung[J]. Int J Radiat Oncol Biol Phys, 2003, 56(1): 106-113. DOI:10.1016/s0360-3016(03)00094-4 |
| [18] |
Willner J, Jost A, Baier K, et al. A little to a lot or a lot to a little? An analysis of pneumonitis risk from dose-volume histogram parameters of the lung in patients with lung cancer treated with 3-D conformal radiotherapy[J]. Strahlenther Onkol, 2003, 179(8): 548-556. DOI:10.1007/s00066-003-1078-0 |
| [19] |
Yorke ED, Jackson A, Rosenzweig KE, et al. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study[J]. Int J Radiat Oncol Biol Phys, 2005, 63(3): 672-682. DOI:10.1016/j.ijrobp.2005.03.026 |
| [20] |
Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes[J]. Int J Radiat Oncol Biol Phys, 2010, 76(1): 287-295. DOI:10.1016/j.ijrobp.2009.05.038 |
| [21] |
Zhang Q, Yu XL, Hu WG, et al. Dosimetric comparison for volumetric modulated arc therapy and intensity-modulated radiotherapy on the left-sided chest wall and internal mammary nodes irradiation in treating post-mastectomy breast cancer[J]. Radiol Oncol, 2015, 49(1): 91-98. DOI:10.2478/raon-2014-0033 |
 2019, Vol. 39
2019, Vol. 39


