尽管我国对适龄女性已经开始接种宫颈癌疫苗,但由于接种率和筛查率依旧明显低于发达国家,且宫颈癌致病潜伏期较长,我国宫颈癌发病率短期内仍会高居不下。放射治疗既可以作为宫颈癌的根治性治疗手段,也可以作为早期宫颈癌术后辅助性治疗手段。而对于中、晚期宫颈癌,国际公认的、标准的治疗方案是同步放化疗,目前已广泛应用于临床。据印度研究报道,宫颈癌放疗3年生存率可达79.4%,与法国、美国和澳大利亚等多个中心的结果相似[1-2]。
然而,仍有一部分宫颈癌患者经过同步放化疗后,出现复发和/或转移而导致死亡。国外研究机构报道[2],宫颈癌根治性放疗后复发或转移的比例在10%~20%,而病情变化的原因不尽相同,这部分复发或者转移患者是宫颈癌临床研究的热点和难点。本研究回顾性分析于吉林省肿瘤医院放疗科行根治性放疗的211例宫颈癌患者临床资料,观察近、远期疗效和相关预后因素,以期总结出复发、转移的危险因素,对宫颈癌个体化治疗提供思路。
资料与方法1.临床资料:筛选并入组吉林省肿瘤医院放疗四科自2014年6月至2017年2月行根治性放疗的宫颈癌患者共211例,既往未手术、无其他部位放疗史,全部病例均经病理确诊。年龄25~82岁,平均年龄(52.8±15.15)岁,中位年龄53.5岁。根据国际妇产科学联盟(FIGO)2009年分期标准,ⅠA/B11例,ⅡA11例,ⅡB 89例,ⅢA7例,ⅢB 93例。盆、腹腔淋巴结转移通过影像学诊断,诊断标准为:CT上短径≥0.8 cm,存在中心低密度坏死区域,或PET-CT提示淋巴结转移。临床详细资料详见表 1。
|
|
表 1 211例宫颈癌患者临床资料 Table 1 General characteristics of 211 patients with cervical cancer |
2.治疗方法:治疗采用体外放疗和腔内放疗相结合的方式。盆腔外照射采用三维适形或调强放疗,总剂量45.0~50.4 Gy,1.8~2.0 Gy/次,盆腔转移淋巴结同步加量至56.0~61.6 Gy,2.0~2.2 Gy/次。外照射后程,CT图像引导下使用荷兰核通公司高剂量率192Ir后装机进行腔内后装放疗,2次/周,5.0~6.0 Gy/次,共4~6次。同步化疗以铂类为基础。
3.随访:所有患者治疗后均定期随访至2019年2月或死亡时截止,中位随访时间35个月(6~55个月)。第1~2年每3个月复查1次,第3~5年每6个月复查1次。总生存时间(overall survival, OS)定义为从治疗开始至任何原因引起死亡或者末次随访的时间,无病生存时间(disease-free survival, DFS)定义为从治疗开始至发生复发、转移以及任何原因引起死亡或者末次随访的时间。
4.统计学处理:使用SPSS 19.0软件进行统计分析,生存分析采用Kaplan-Meier法,单因素分析采用Log-rank检验进行,多因素分析采用COX比例风险回归模型进行,P<0.05为差异有统计学意义。
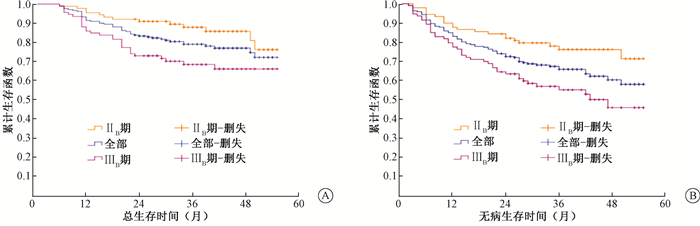
结果1.复发、转移及生存情况:治疗结束后,完全缓解(CR)率达91%,疗效评价均为影像学证据与妇科检查相结合。211例患者随访期间共46例死亡,其中,2例非肿瘤相关死亡,1例第二原发肿瘤结肠癌导致死亡,12例肿瘤相关死亡但具体情况不详;复发转移57例,其中,单纯局部区域复发16例(死亡10例,62.5%),单纯远处转移27例(死亡10例,37.0%),局部区域复发合并远处转移14例(死亡11例, 78.6%),相应部位见表 2,3;2例第二原发肿瘤肺腺癌仍存活。全组患者2年OS、DFS为83.4%、72.5%,占比重较大的Ⅱ B和Ⅲ B期宫颈癌患者2年OS、DFS分别为91%、82%和73.1%、63.4%(图 1)。
|
|
表 2 211例宫颈癌患者放疗后远处转移情况 Table 2 Distant metastasis after radiotherapy in 211 patients with cervical cancer |
|
|
表 3 211例宫颈癌患者放疗后局部复发情况 Table 3 Local recurrence after radiotherapy in 211 patients with cervical cancer |
|
图 1 211例宫颈癌患者根治性放疗后总生存曲线(A)和无病生存曲线(B) Figure 1 OS (A)and DFS (B) curves after radiotherapy in 211 patients of cervical cancer |
2.预后影响因素分析:单因素分析显示,宫颈癌患者的2年OS、DFS与病理类型、治疗前鳞状细胞抗原(SCC)值、FIGO分期明显相关(OS: χ2=7.123, 6.014和8.398, P<0.05; DFS: χ2=11.832, 8.003和7.731, P<0.05),除了以上因素外,2年DFS还与盆腔淋巴结转移有关(χ2=9.286, P<0.05, 表 4)。
|
|
表 4 211例宫颈癌患者预后单因素分析(%) Table 4 Univariate analysis of prognosisin 211 patients with cervical cancer(%) |
将单因素分析得出的结果纳入多因素分析,结果列于表 5。由表 5可知,病理类型(HR =2.963,95%CI:1.020~8.607,P<0.05)、治疗前SCC值(HR =2.473,95%CI:1.132~5.405,P<0.05)、分期(HR=2.574,95%CI:1.222~5.423,P<0.05)是影响OS的独立预后因素;影响DFS的独立预后因素包括病理类型(HR =3.014,95%CI:1.321~6.878,P<0.05)、治疗前SCC值(HR =1.988,95%CI:1.033~3.828,P<0.05)及盆腔淋巴结是否转移(HR =1.914,95%CI:1.080~3.392,P<0.05)。
|
|
表 5 211例宫颈癌患者预后多因素分析 Table 5 Multivariate analysis of prognosis in 211 patients with cervical cancer |
讨论
宫颈癌是一种高复发率的疾病,且大多数病情进展发生在2~3年内。据统计,宫颈癌放疗后失败的患者中,盆腔内复发占70%,复发患者中发生远处转移的超过50%[2-3]。本研究中,复发30例,占所有入组病例的52.6%,且46.7%(14/30)的复发患者发生了远处转移,与上述结论相接近。预后相关因素的确定对于可能受益于新治疗策略的患者非常重要。本文回顾性研究表明,宫颈癌根治性放疗后CR率达90%以上,2年OS和DFS分别达83.4%、72.5%,疗效基本满意。多因素分析显示,病理类型、治疗前SCC水平和分期都是降低OS的危险因素,DFS的影响因素与OS相比,剔除了分期,增加了盆腔淋巴结因素。
诸多研究表明,FIGO分期和肿瘤大小是独立的预后影响因素,分期越晚、肿瘤越大,预后越差[4-5]。美国妇科肿瘤学组(GOG)一项回顾性分析的结果中[6],二者为宫颈癌放疗后盆腔复发最重要的两个危险因素。Queiroz等[7]报道,肿瘤≥6 cm与局部复发风险增加相关,同时也是导致OS恶化的唯一因素。本研究多因素分析结果显示,FIGO分期对OS的影响比对DFS的影响更强(P=0.013,P=0.064), 大肿瘤(≥4 cm)有降低OS及DFS的趋势,但差异无统计学意义(P=0.081,P=0.052)。2018年末宫颈癌FIGO新分期将盆腔和腹主动脉旁淋巴结转移列入新增的Ⅲ C期,明确了淋巴结转移显著影响宫颈癌的预后[8]。既往研究提示,盆腔淋巴结转移是中晚期宫颈癌放疗后远处转移的危险因素,能够使OS下降35%~40%[9-11]。本研究结果与上述研究略有出入,盆腔淋巴结转移对OS的影响无统计学意义,但能显著影响治疗后的DFS(P=0.026),据此在制定治疗方案前必须全面评估淋巴结转移状态,临床分期早但淋巴结有转移的患者可能需要更加积极的治疗,如巩固化疗等。
Kato和Torigoe[12]发现,血清SCC抗原水平是监测宫颈鳞癌疗效的有效标志物。研究者们在此基础上继续探索,确定其与宫颈癌患者放化疗后的复发和死亡率密切相关,可用于监测病情进展[13-16]。Lekskul等[17]报道了治疗前血清SCC-Ag水平与肿瘤大小明确相关间接影响预后;Kim等[18]还发现治疗后SCC的水平也与远处转移关系密切。本研究比较的是治疗前血清SCC-Ag水平,发现其与OS和DFS均显著相关,是影响宫颈癌患者放疗预后重要的独立因素。病理类型是另一比较重要的危险因子。Gien等[19]研究表明,宫颈腺癌患者预后差,与鳞癌相比,5年生存率降低10%~20%,且有更加明显的转移倾向[20]。与之结果相类似,北京协和医院针对两种病理类型进行了对比研究[21],接受根治性放疗的宫颈鳞癌与腺癌患者3年总生存率分别为85.2%和75.4% (P=0.005),并且生存率在单纯放疗和同步放化疗无明显差异。Xiong等[22]还发现腺癌放疗后疗效达CR需要相对于鳞癌更长的时间,总生存率和局控率也更差一些,推测可能与腺癌放射敏感性低有关。本研究结果中,单因素和多因素一致表明腺癌是宫颈癌预后的独立危险因素,显著降低OS和DFS,提示宫颈腺癌需要采取更加有效的治疗方案,如诱导化疗和巩固化疗等。一项临床试验将880例FIGO期Ⅱ B~ⅣA宫颈腺癌患者随机分为同步放化疗组和同步放化疗+1个周期新辅助化疗+2个周期巩固化疗组,后者DFS、累积生存率以及局控率都明显升高(P<0.05)[23],说明积极的辅助化疗对改善宫颈腺癌生存率有效。
综上,本研究病例2年OS为83.4%,肿瘤病理类型、治疗前SCC水平及临床分期是影响患者生存率的独立预后因素,腺癌、SCC≥30 ng/ml、局部晚期宫颈癌患者预后较差。明确和了解预后危险因素,对于复发或转移高风险患者,更应在是否巩固化疗、同步放化联合靶向治疗,以及放射靶区设计和追加照射剂量等方面提供更具个体化的综合治疗策略。
利益冲突 无作者贡献声明 修雨婷负责资料收集与数据分析,撰写论文;孟凡旭负责随访;欧健负责修改论文;王琢、杜晶负责协助处理数据分析;赵康康、王蕴龙协助随访资料的整理;陈志深、田琦协助查阅相关文献;孙宝胜负责研究设计和论文修改
| [1] |
Tiwari R, Narayanan GS, Jayakumar V, et al. The promise of image-guided brachytherapy of better clinical outcomes in treatment of cervical cancer:Does it deliver? An Indian scenario[J]. Gynecol Oncol, 2018, 150(3): 420-425. DOI:10.1016/j.ygyno.2018.07.012 |
| [2] |
Mahantshetty U, Krishnatry R, Hande V, et al. Magnetic resonance image guided adaptive brachytherapy in locally advanced cervical cancer:an experience from a tertiary cancer center in a low and middle income countries setting[J]. Int J Radiat Oncol Biol Phys, 2017, 99(3): 608-617. DOI:10.1016/j.ijrobp.2017.06.010 |
| [3] |
Kawaguchi R, Furukawa N, Kobayashi H, et al. Posttreatment cut-off levels of squamous cell carcinoma antigen as a prognostic factor in patients with locally advanced cervical cancer treated with radiotherapy[J]. J Gynecol Oncol, 2013, 24(4): 313-320. DOI:10.3802/jgo.2013.24.4.313 |
| [4] |
Intaraphet S, Kasatpibal N, Søgaard M, et al. Histological type-specific prognostic factors of cervical small cell neuroendocrine carcinoma, adenocarcinoma, and squamous cell carcinoma[J]. Onco Targets Ther, 2014, 7: 1205-1214. DOI:10.2147/OTT.S64714 |
| [5] |
Kim TE, Park BJ, Kwack HS, et al. Outcomes and prognostic factors of cervical cancer after concurrent chemoradiation[J]. J Obstet Gynaecol Res, 2012, 38(11): 1315-1320. DOI:10.1111/j.1447-0756.2012.01871.x |
| [6] |
Rose PG, Java J, Whitney CW, et al. Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG Oncology/Gynecologic Oncology Group randomized trials of chemoradiotherapy[J]. J Clin Oncol, 2015, 33(19): 2136-2142. DOI:10.1200/JCO.2014.57.7122 |
| [7] |
Queiroz ACM, Fabri V, Mantoan H, et al. Risk factors for pelvic and distant recurrence in locally advanced cervical cancer[J]. Eur J Obstet Gynecol Reprod Biol, 2019, 235: 6-12. DOI:10.1016/j.ejogrb.2019.01.028 |
| [8] |
Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri[J]. Int J Gynecol Obstet, 2018, 143(Suppl 2): 22-36. DOI:10.1002/ijgo.12611 |
| [9] |
Parker K, Gallop-Evans E, Hanna L, et al. Five years' experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy:results from a single institution[J]. Int J Radiat Oncol Biol Phys, 2009, 74(1): 140-146. DOI:10.1016/j.ijrobp.2008.06.1920 |
| [10] |
Atahan IL, Onal C, Ozyar E, et al. Long-term outcome and prognostic factors in patients with cervical carcinoma:a retrospective study[J]. Int J Gynecol Cancer, 2007, 17(4): 833-842. DOI:10.1111/j.1525-1438.2007.00895.x |
| [11] |
Hong JH, Tsai CS, Lai CH, et al. Risk stratification of patients with advanced squamous cell carcinoma of cervix treated by radiotherapy alone[J]. Int J Radiat Oncol Biol Phys, 2005, 63(2): 492-499. DOI:10.1016/j.ijrobp.2005.02.012 |
| [12] |
Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma[J]. Cancer, 1977, 40(4): 1621-1638. DOI:10.1002/1097-0142(197710)40:4<1621::AID-CNCR2820400435>3.0.CO;2-I |
| [13] |
Li J, Wu MF, Lu HW, et al. Pretreatment serum lactate dehydrogenase is an independent prognostic factor for patients receiving neoadjuvant chemotherapy for locally advanced cervical cancer[J]. Cancer Med, 2016, 5(8): 1863-1872. DOI:10.1002/cam4.779 |
| [14] |
Kudaka W, Nagai Y, Toita T, et al. Long-term results and prognostic factors in patients with stage Ⅲ-ⅣA squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy from a single institution study[J]. Int J Clin Oncol, 2013, 18(5): 916-921. DOI:10.1007/s10147-012-0457-x |
| [15] |
Kim TE, Park BJ, Kwack HS, et al. Outcomes and prognostic factors of cervical cancer after concurrent chemoradiation[J]. J Obstet Gynaecol Res, 2012, 38(11): 1315-1320. DOI:10.1111/j.1447-0756.2012.01871.x |
| [16] |
Oh J, Lee HJ, Lee TS, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with locally advanced cervical cancer treated with radiation or chemoradiation[J]. Obstet Gynecol Sci, 2016, 59(4): 269-278. DOI:10.5468/ogs.2016.59.4.269 |
| [17] |
Lekskul N, Charakorn C, Lertkhachonsuk AA, et al. The level of squamous cell carcinoma antigen and lymph node metastasis in locally advanced cervical cancer[J]. Asian Pac JCancerPrev, 2015, 16(11): 4719-4722. DOI:10.7314/apjcp.2015.16.11.4719 |
| [18] |
Kim TE, Park BJ, Kwack HS, et al. Outcomes and prognostic factors of cervical cancer after concurrent chemoradiation[J]. J Obstet Gynaecol Res, 2012, 38(11): 1315-1320. DOI:10.1111/j.1447-0756.2012.01871.x |
| [19] |
Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma:a unique cervical cancer[J]. Gynecol Oncol, 2010, 116(1): 140-146. DOI:10.1016/j.ygyno.2009.09.040 |
| [20] |
Kang S, Nam BH, Park JY, et al. Risk assessment tool for distant recurrence after platinum-based concurrent chemoradiation in patients with locally advanced cervical cancer:a Korean Gynecologic Oncology Group Study[J]. J Clin Oncol, 2012, 30(19): 2369-2374. DOI:10.1200/JCO.2011.37.5923 |
| [21] |
Hu K, Wang W, Liu X, et al. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma of cervix after definitive radiotherapy or concurrent chemoradiotherapy[J]. Radiat Oncol, 2018, 13(1): 249. DOI:10.1186/s13014-018-1197-5 |
| [22] |
Xiong Y, Liu J, Chen S, et al. Combination of external beam radiotherapy and californium (Cf)-252 neutron intracavity brachytherapy is more effective in control of cervical squamous cell carcinoma than that of cervical adenocarcinoma[J]. Med Oncol, 2015, 32(9): 231. DOI:10.1007/s12032-015-0670-3 |
| [23] |
Tang J, Tang Y, Yang J, et al. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma[J]. Gynecol Oncol, 2012, 125(2): 297-302. DOI:10.1016/j.ygyno.2012.01.033 |
 2019, Vol. 39
2019, Vol. 39


