2. 473000 南阳市中心医院/郑州大学附属南阳医院放疗科;
3. 473000 南阳市中心医院/郑州大学附属南阳医院肿瘤内科;
4. 473000 南阳市中心医院/郑州大学附属南阳医院重症医学科;
5. 430022 武汉, 华中科技大学同济医学院附属协和医院胰腺外科
2. Department of Radiotherapy, Nanyang City Central Hospital/Nanyang Hospital Affiliated to Zhengzhou University, Nanyang 473000, China;
3. Department of Oncology, Nanyang City Central Hospital/Nanyang Hospital Affiliated to Zhengzhou University, Nanyang 473000, China;
4. Department of Critical Care Medicine, Nanyang City Central Hospital/Nanyang Hospital Affiliated to Zhengzhou University, Nanyang 473000, China;
5. Department of Pancreatic Surgery, Wuhan Union Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China
胰腺癌是导致癌症相关死亡的第7大原因[1-2]。尽管现代医学技术有所进步,胰腺癌患者生存状况有所改善,但其5年总生存率仍仅为6%,中位生存期仅为9个月[3-4]。固有的和获得性放化疗抵抗行为,是显著降低胰腺癌放化疗临床疗效的主要因素[5]。因此,更好地了解胰腺癌放化疗抵抗的分子机制有助于其临床治疗。序列相似性家族83成员A(family with sequence similarity 83, member A,FAM83A)位于染色体8q24上,最初通过生物信息学方法被鉴定为潜在的肿瘤特异性基因[6]。FAM83A在多种人类肿瘤中高表达,包括肺癌、乳腺癌、睾丸癌和膀胱癌[7-8],表明FAM83A在癌症发生进展过程中可能作为致癌基因发挥作用。然而,FAM83A在胰腺癌细胞放化疗敏感性中的作用少见报道。因此,本研究将通过慢病毒方法构建稳定沉默FAM83 A的胰腺癌PANC-1细胞株,研究沉默FAM83A后PANC-1细胞对胰腺癌临床化疗药物吉西他滨及放射敏感性的变化,以期揭示FAM83A在胰腺癌进展中的作用,为临床靶向治疗提供新靶标。
材料与方法1.材料:胰腺癌细胞PANC-1购自中国科学院上海细胞库;青链霉素、DMEM培养基、Lipofectamine 2000、TRIzol购自美国Life公司;胎牛血清购自美国GIBCO公司;FAM83A shRNA由上海生工生物公司合成;PrimeScriptTM RT reagent Kit with gDNA Eraser、SYBR®Premix Ex TaqTM购自大连TaKaRa公司;嘌呤霉素、RIPA裂解液、二喹啉甲酸(BCA)蛋白定量试剂盒、Annexin V-FITC细胞凋亡检测试剂盒购自上海碧云天公司;抗FAM83A抗体购自美国Sigma公司;active-β-肌动蛋白购自德国EMD Millipore公司;Total β-肌动蛋白、p-β-肌动蛋白、GAPDH购自美国Cell Signaling Technology公司;二抗、ECL购自德国Merck Millipore公司;PE-CD133抗体购自ThermoFisher Scientific公司;吉西他滨购自西班牙Lilly公司。
2.细胞培养和稳转株构建:胰腺癌细胞PANC-1用含10%胎牛血清、1%双抗的DMEM培养基于37℃、5% CO2潮湿大气的细胞培养箱中培养。将FAM83A的shRNA序列(5′ GCACAACAACATCAGAGACCT 3′)克隆至pLKO.1-puro中构建沉默FAM83A的慢病毒载体,并与包装质粒共转至293T细胞包装慢病毒,收集病毒过滤,-80℃保存备用。将收集的慢病毒感染PANC-1细胞20 h,换新鲜培养液,继续培养至48 h,用0.5 mg/ml嘌呤霉素筛选得稳定沉默FAM83A的PANC-1稳转株。
3. qPCR检测:取上述PANC-1稳转株用TRIzol提取总RNA,用PrimeScriptTM RT reagent Kit with gDNA Eraser试剂盒进行反转录,采用SYBR®Premix Ex TaqTM试剂盒进行qPCR检测FAM83A的表达,以GAPDH为内参,用2-ΔΔCt法来计算基因相对表达量。基因引物如下:FAM83A:正向引物5′CCCATCTCAGTCACTGGCATT 3′和反向引物5′CCGCCAACATCTCCTTGTTC 3′;GAPDH:正向引物5′AATGAAGGGGTCATTGATGG 3′和反向引物5′AAGGTGAAGGTCGGAGTCAA 3′。
4.Western blot检测:取上述PANC-1稳转株用RIPA裂解液冰上裂解细胞30 min,12 000 g,离心半径5 cm,离心15 min,收集上清液经BCA法测蛋白浓度。取适量蛋白样品用12% SDS-PAGE凝胶电泳分离蛋白并将分离蛋白样转至聚偏二氟乙烯(polyvinylidene fluoride,PVDF)膜上,5%脱脂牛奶室温封闭2 h,FAM83A、Active-β-肌动蛋白、p-β-肌动蛋白、Total β-肌动蛋白、GAPDH一抗稀释液4℃孵育过夜,用含有吐温20的三羟甲基氨基甲烷缓冲盐液(TBST)洗涤3次,相应二抗稀释液室温孵育2 h,TBST洗涤3次,加适量增强化学发光液(enhanced chemiluminescence,ECL)于Western blot仪曝光显影,以GAPDH为内参,Image J软件对蛋白条带进行定量。
5.流式细胞仪检测CD133阳性的PANC-1细胞含量:取对数生长期的PANC-1稳转株,用预冷的无钙镁的磷酸盐缓冲液(PBS)洗涤2次,0.25%胰蛋白酶消化,取1×106细胞置于流式管中,用2 ml 4%多聚甲醛室温固定40 min,然后12 000 g,离心半径5 cm,离心15 min,用PBS洗涤2次,加入200 μl含PE-CD133抗体的PBS重悬细胞,室温避光孵育30 min,离心用含0.5 %牛血清白蛋白(BSA)的PBS重悬,流式细胞仪检测。
6. PANC-1细胞肿瘤干细胞球形成实验:取500个对数生长期的PANC-1稳转细胞接种于含0.4%BSA、20 ng/ml EGF、20 ng/ml bFGF、2% B27和5 μg/ml胰岛素的DMEM-F12培养基中,于6孔板中培养约10~14 d,显微镜下计数干细胞球形成数目,计算干细胞球形成率。
7.细胞活力检测:取3 000个对数生长期细胞接种于96孔板中,设空白对照组、阴性对照组、吉西他滨组、沉默FAM83A组、沉默FAM83A+吉西他滨组,每组设3个复孔。待细胞贴壁后,吉西他滨组和shFAM83A+吉西他滨组分别加入50 μmol/L的吉西他滨处理细胞24 h,每孔加入20 μl 5 mg/ml的四甲基偶氮唑盐(MTT),继续孵育4 h,弃上清每孔加入150 μl二甲基亚砜(DMSO),酶标仪上振荡10 min,测570 nm波长下各孔吸光度(A)值。
8.细胞凋亡检测:用不含EDTA的胰蛋白酶消化收集细胞,冷的PBS洗涤2次,取5×105个细胞加入500 μl结合缓冲液(binding buffer)重悬细胞,然后加入5 μl的Annexin V-FITC和5 μl的PI混匀,室温避光孵育15 min,流式细胞仪检测细胞凋亡。
9.克隆形成检测:取处理后细胞3 000个接种于6孔板中,过夜贴壁后,接受2 Gy的X射线照射,然后置于细胞培养箱中继续培养,14 d后取出,弃去旧培养液,PBS洗3次,甲醇固定10 min,晾干后0.1%结晶紫染色,双蒸水洗去残留结晶紫,晾干后于显微镜下观察并拍照,计数各孔克隆数,只计单个多于50个细胞的克隆。照射条件如下:6 MV X射线,源靶距(SSD)为100 cm,照射野10 cm×10 cm,剂量DT=2 Gy,剂量率为2.59 cGy/h,射野覆盖全细胞培养板。
10.统计学处理:实验数据用x±s表示。采用SPSS 22.0软件进行分析。两组数据比较经正态性检验符合正态分布。采用独立样本t检验,多组差异比较经方差齐性检验后采用单因素方差分析,组间比较采用LSD-t检验。P < 0.05为差异有统计学意义。
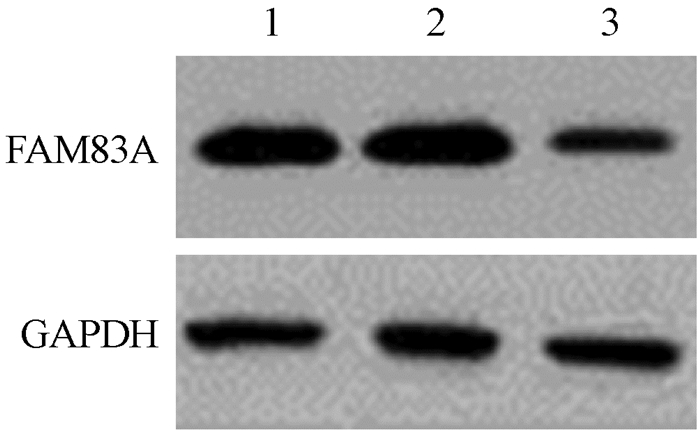
结果1.构建稳定沉默FAM83A的PANC-1细胞株:为进一步研究FAM83A对胰腺癌细胞辐射敏感性的影响,本研究通过慢病毒感染方法构建了稳定沉默FAM83A的PANC-1细胞株。经qPCR和Western blot检测发现,与阴性对照组相比,沉默FAM83A后PANC-1中FAM83A蛋白和mRNA水平均显著降低(t=9.410、12.600,P < 0.05,图 1,表 1),表明稳定沉默FAM83A的PANC-1细胞株构建成功。
|
图 1 PANC-1细胞中FAM83A蛋白表达 注:1.空白对照组;2.阴性对照组;3.沉默FAM83A组 Figure 1 FAM83A protein in PANC-1 cells |
|
|
表 1 PANC-1细胞中FAM83A蛋白和mRNA相对表达水平(x±s) Table 1 Relative protein and mRNA levels of FAM83A in PANC-1 cells(x±s) |
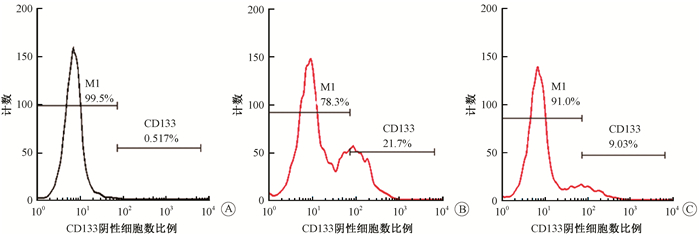
2.沉默FAM83A对胰腺癌PANC-1细胞干细胞样表型的影响:流式细胞仪检测阴性对照组和沉默FAM83A组CD133阳性细胞率。结果如图 2、表 2所示,沉默FAM83A后CD133阳性PANC-1细胞率较阴性对照组显著降低(t=8.184,P < 0.05);且沉默FAM83A后细胞成球能力较阴性对照组也显著降低(t=9.311,P < 0.05),表明沉默FAM83A抑制胰腺癌PANC-1细胞干细胞样表型。
|
图 2 流式细胞仪检测CD133阳性PANC-1细胞数A.空白对照组;B.阴性对照组;C.沉默FAM83A组 注:M1.阴性对照峰 Figure 2 The number of CD133+ PANC-1 cells were detected by flow cytometer A. Isotype control group; B. Negative control group; C. Silenced FAM83A group |
|
|
表 2 PANC-1细胞中CD133阳性细胞数和细胞成球变化(x±s) Table 2 The number of CD133+ cells and the cell globular changes in PANC-1 cells(x±s) |
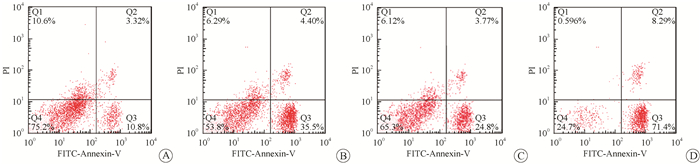
3.沉默FAM83A增加PANC-1细胞对吉西他滨的敏感性:利用50 μmol/L的吉西他滨处理PANC-1细胞72 h,MTT检测发现,与阴性对照组相比,吉西他滨组、沉默FAM83A组、沉默FAM83A联合吉西他滨组均可显著抑制细胞活力(t=6.887、5.024、15.150,P < 0.05),沉默FAM83A联合吉西他滨后的细胞活力较吉西他滨处理后的细胞活力明显降低(t=6.378,P < 0.05)(表 3)。流式细胞仪检测细胞凋亡结果表明,与阴性对照组相比,吉西他滨组、沉默FAM83A组、沉默FAM83A联合吉西他滨组细胞凋亡均显著增加(t=9.082、6.725、12.810,P < 0.05),沉默FAM83A联合吉西他滨后的细胞活力较吉西他滨处理后的细胞凋亡显著增加(t=7.929,P < 0.05)(图 3、表 3),由此可知,沉默FAM83A可增加胰腺癌PANC-1细胞对吉西他滨敏感性。
|
图 3 流式细胞仪检测吉西他滨处理PANC-1稳转株后细胞凋亡变化A.阴性对照组;B.吉西他滨组;C.沉默FAM83A组;D.沉默FAM83A联合吉西他滨组 Figure 3 The apoptosis rate of PANC-1 cells with stable silencing FAM83A after treatment of gemcitabine was detected by flow cytometer A. Negative control group; B. Gemcitabine group; C. Silenced FAM83A group; D. Silenced FAM83A combined with gemcitabine group |
|
|
表 3 PANC-1细胞活力和凋亡变化(x±s) Table 3 The cell viability and apoptosis rate of PANC-1 cells(x±s) |
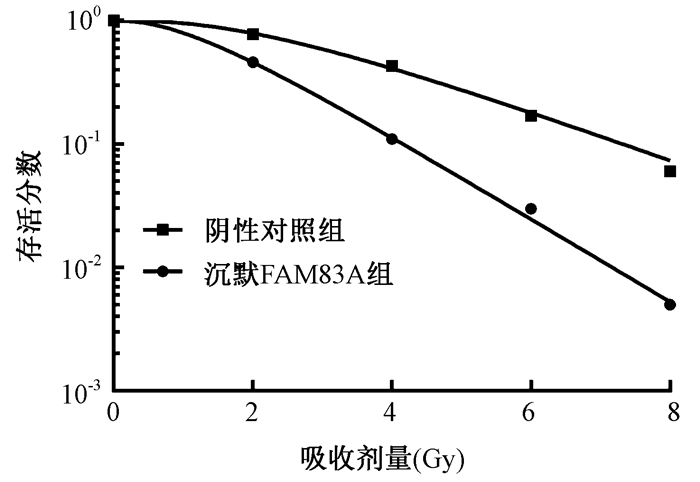
4.沉默FAM83A对PANC-1细胞辐射敏感性的影响:存活分数结果表明,Scrambled+IR组和shFAM83A+IR组的SF2、D0、Dq分别为79.120、2.150、2.448和45.960、1.293、1.220,shFAM83A+IR组的放射增敏比(SER)为1.662(图 4)。经2 Gy X射线照射后,与阴性对照组相比,X射线照射组、沉默FAM83A组、沉默FAM83A联合X射线照射组细胞克隆形成能力均显著降低(t=3.599、5.934、12.200,P < 0.05),细胞凋亡率显著增加(t=10.450、11.530、13.630,P < 0.05);且沉默FAM83A联合X射线照射组较X射线照射组细胞活力明显降低,细胞凋亡率明显增加(t=6.694、8.990,P < 0.05,表 4),由上述结果可知,沉默FAM83A可增加PANC-1细胞对放射的敏感性。
|
图 4 沉默FAM83A增加PANC-1细胞对X射线照射敏感性 Figure 4 Silencing FAM83A increases radiosensitivity of PANC-1 cells |
|
|
表 4 PANC-1细胞克隆形成和凋亡变化(x±s) Table 4 Cell colony formation and apoptosis rate of PANC-1 cells(x±s) |
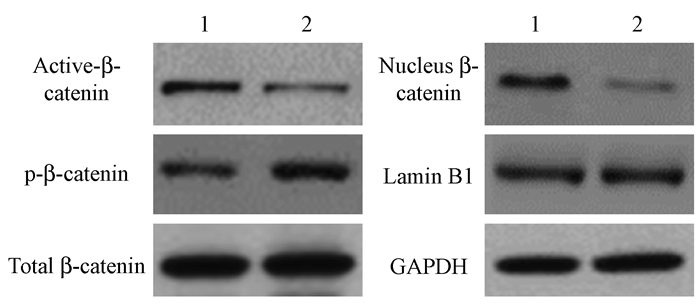
5.沉默FAM83A抑制PANC-1细胞中Wnt/β-catenin信号通路:结果如图 5、表 5所示,与阴性对照组相比,沉默FAM83A后PANC-1细胞中激活的β-catenin(Active-β-catenin)表达显著降低,磷酸化的β-catenin(p-β-catenin)表达明显增加,且细胞核中β-catenin表达也显著降低(t=10.290、8.521、8.969,P < 0.05),而总的β-catenin(Total β-catenin)无明显变化。由此得知,沉默FAM83A能够抑制PANC-1细胞中Wnt/β-catenin信号转导,提示在胰腺癌细胞中FAM83A可能是通过Wnt/β-catenin信号来发挥作用。
|
图 5 Western blot检测沉默FAM83A对PANC-1细胞中Wnt/β-catenin信号蛋白表达影响 注:1.阴性对照组;2.沉默FAM83A组 Figure 5 Effect of silencing FAM83A on Wnt/β-catenin expression in PANC-1 cells |
|
|
表 5 沉默FAM83A后PANC-1细胞中Wnt/β-catenin信号蛋白相对表达水平(x±s) Table 5 The relative expression levels of Wnt/β-catenin signaling proteins in PANC-1 cells after silencing FAM83A (x±s) |
讨论
胰腺癌是一种具有高度侵袭性的恶性肿瘤,发病隐匿,大部分患者确诊时已属中晚期,5年生存率仅有1%~4%,预后极差[9],深入研究胰腺癌的发病机制对提高胰腺癌的早期诊断及改善预后非常重要。据研究报道,吉西他滨、厄洛替尼(erlotinib)和5-氟尿嘧啶(5-FU)等常用化疗药物对胰腺癌的缓解率低于25%[5, 10-11],固有的和获得性放化疗抵抗行为是显著降低胰腺癌放化疗临床疗效的主要因素[5]。
FAM83A是一个新的癌基因,潜在的靶点[7],最初通过使用两个独特的正向遗传筛选,由Jackson和Bissell实验室发现的一个新的致癌基因家族,被命名为FAM83A-H,其可使人类乳腺上皮细胞(HMEC)发生转化[12-13]。Lee等[13]发现FAM83A可能通过与c-RAF和磷脂酰肌醇3激酶p85相互作用激活EGFR/PI3K/AKT信号通路,从而使得乳腺癌对酪氨酸激酶抑制剂(tyrosine kinase inhibitors,TKIs)产生抗性[14],表明高表达的FAM83A可能导致细胞具有抗性。许亚娜等[15]研究发现,FAM83A是一种新的检测乳腺癌外周血循环癌细胞的肿瘤标记物, 并可作为判断乳腺癌转移和预后的评估指标。FAM83A在乳腺癌组织中高表达,而在正常人体组织中几乎不表达[16]。此外,李泓漪等[17]发现FAM83B可能参与食管鳞癌的发生、发展和浸润,FAM83B的表达水平可能成为食管鳞癌预后强有力的预测因子,进一步表明FAM83A可能是癌症的潜在治疗靶点。在本研究中也发现,沉默FAM83A可显著抑制胰腺癌细胞PANC-1增殖、克隆形成,并促进细胞凋亡。
癌症干细胞(CSCs),也称为肿瘤起始细胞(TICs)对传统治疗具有内在抗性,目前被认为是潜在的治疗靶点[18]。最近有报道指出,在卵巢癌中治疗失败及卵巢癌耐药性与其中CSCs积累密切相关[19]。Li等[20]研究发现,化疗药物如多西紫杉醇、多柔比星或环磷酰胺治疗的乳腺癌患者中,对化疗具有抗性的CD44阳性CD24- /low CSC亚群的细胞百分比明显增加。此外,CD133阳性胰腺癌CSCs被证实是高致瘤的,对放化疗均具有高度抗性,并且CD133阳性CXCR4阳性胰腺癌CSCs亚群是肿瘤转移的关键[21-22]。因此,干扰胰腺癌干细胞可能会增加其对放化疗敏感性,从而改善对治疗效果。本研究发现,沉默FAM83A后CD133阳性的PANC-1细胞及细胞成球能力均显著降低,干细胞特性被显著抑制,这与先前结果一致。此外,本研究还发现,沉默FAM83A后PANC-1细胞对化疗药物吉西他滨及放射敏感性显著增加,与肿瘤恶性相关的Wnt/β-catenin信号通路被显著抑制。
综上所述,本研究发现沉默FAM83A可能通过Wnt/β-catenin信号通路显著抑制胰腺癌PANC-1细胞干细胞样表型,增强细胞对化疗药物吉西他滨及放射敏感性,表明FAM83A将可成为胰腺癌临床靶向治疗的潜在新靶点。
利益冲突 全体作者无利益冲突,排名无争议,未因进行该研究而接受任何不正当的职务和财务利益作者贡献声明 倪猛负责实验和论文撰写;殷涛、万里新负责数据统计;王旸、郑喜胜协助完成实验;樊宏伟参与研究设计与指导,提出修改意见
| [1] |
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide:sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): E359-386. DOI:10.1002/ijc.29210 |
| [2] |
Wei QC, Yu W, Rosati LM, et al. Advances of stereotactic body radiotherapy in pancreatic cancer[J]. Chin J Cancer Res, 2015, 27(4): 349-357. DOI:10.3978/j.issn.1000-9604.2015.04.12 |
| [3] |
Ilmer M, Boiles AR, Regel I, et al. RSPO2 enhances canonical Wnt signaling to confer stemness-associated traits to susceptible pancreatic cancer cells[J]. Cancer Res, 2015, 75(9): 1883-1896. DOI:10.1158/0008-5472.CAN-14-1327 |
| [4] |
金子良, 王理伟. 胰腺癌的发病机制和内科治疗的进展[J]. 上海医学, 2014, 37(3): 196-199. Jin ZL, Wang LW. The pathogenesis of pancreatic cancer and the progress of medical treatment[J]. Shanghai Med J, 2014, 37(3): 196-199. DOI:10.3969/j.issn.0253-9934.2014.03.018 |
| [5] |
Singh SK, Chen NM, Hessmann E, et al. Antithetical NFATc1-Sox2 and p53-miR200 signaling networks govern pancreatic cancer cell plasticity[J]. EMBO J, 2015, 34(4): 517-530. DOI:10.15252/embj.201489574 |
| [6] |
Chen S, Huang J, Liu Z, et al. FAM83A is amplified and promotes cancer stem cell-like traits and chemoresistance in pancreatic cancer[J]. Oncogenesis, 2017, 6(3): e300. DOI:10.1038/oncsis.2017.3 |
| [7] |
Bartel CA, Jackson MW. HER2-positive breast cancer cells expressing elevated FAM83A are sensitive to FAM83A loss[J]. PLoS One, 2017, 12(5): e0176778. DOI:10.1371/journal.pone.0176778 |
| [8] |
Snijders AM, Lee SY, Hang B, et al. FAM83 family oncogenes are broadly involved in human cancers:an integrative multi-omics approach[J]. Mol Oncol, 2017, 11(2): 167-179. DOI:10.1002/1878-0261.12016 |
| [9] |
张创杰, 张连峰, 周琳. 自噬相关蛋白Beclin1、LC3和P62在进展期胰腺癌中的表达及临床意义[J]. 世界华人消化杂志, 2015, 23(2): 318-323. Zhang CJ, Zhang LF, Zhou L. Clinical significance of expression of autophagy-related proteins Beclin1, LC3 and P62 in advanced pancreatic cancer[J]. World Chin J Digestol, 2015, 23(2): 318-323. DOI:10.3969/acjd.v23.i2.318 |
| [10] |
Li J, Wientjes MG, Au JL. Pancreatic cancer:pathobiology, treatment options, and drug delivery[J]. AAPS J, 2010, 12(2): 223-232. DOI:10.1208/s12248-010-9181-5 |
| [11] |
Oberstein PE, Olive KP. Pancreatic cancer:why is it so hard to treat?[J]. Therap Adv Gastroenterol, 2013, 6(4): 321-337. DOI:10.1177/1756283X13478680 |
| [12] |
Cipriano R, Graham J, Miskimen KLS, et al. FAM83B mediates EGFR-and RAS-driven oncogenic transformation[J]. J Clin Invest, 2012, 122(9): 3197-3210. DOI:10.1172/JCI60517 |
| [13] |
Lee SY, Meier R, Furuta S, et al. FAM83A confers EGFR-TKI resistance in breast cancer cells and in mice[J]. J Clin Invest, 2012, 122(9): 3211-3220. DOI:10.1172/JCI60498 |
| [14] |
Grant S. FAM83A and FAM83B:candidate oncogenes and TKI resistance mediators[J]. J Clin Invest, 2012, 122(9): 3048-3051. DOI:10.1172/JCI64412 |
| [15] |
许亚娜, 许倩, 刘镭. 乳腺癌外周血标记物FAM83A的检测及临床意义[J]. 河北医学, 2013, 19(10): 1446-1450. Xu YN, Xu Q, Liu L. Detection of a tumor marker FAM83A for circulating cancer of breast cancer and its clinical significance[J]. Hebei Med, 2013, 19(10): 1446-1450. DOI:10.3969/j.issn.1006-6233.2013.10.003 |
| [16] |
Bartel CA, Parameswaran N, Cipriano R, et al. FAM83 proteins:Fostering new interactions to drive oncogenic signaling and therapeutic resistance[J]. Oncotarget, 2016, 7(32): 52597-52612. DOI:10.18632/oncotarget.9544 |
| [17] |
李泓漪, 程彩霞, 翟元芳, 等. FAM83B的表达与食管鳞癌临床病理特征及预后的相关性[J]. 山西医科大学学报, 2017, 48(6): 583-588. Li HY, Cheng CX, Zhai YF, et al. Expression of FAM83B in esophageal squamous cell carcinoma and its association with clinicopathological characteristics and prognosis[J]. J Shanxi Med Univ, 2017, 48(6): 583-588. DOI:10.13753/j.issn.1007-6611.2017.06.016 |
| [18] |
Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells-what challenges do they pose?[J]. Nat Rev Drug Discov, 2014, 13(7): 497-512. DOI:10.1038/nrd4253 |
| [19] |
尹晓龙, 李燕华. 肿瘤干细胞与卵巢癌耐药的研究进展[J]. 中华全科医学, 2016, 14(2): 288-290. Yin XL, Li YH. Research progress of drug resistance in cancer stem cells and ovarian cancer[J]. Chin J Gen Pract, 2016, 14(2): 288-290. DOI:10.16766/j.cnki.issn.1674-4152.2016.02.044 |
| [20] |
Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy[J]. J Natl Cancer Inst, 2008, 100(9): 672-679. DOI:10.1093/jnci/djn123 |
| [21] |
王花, 李雯, 汪谦. CD133在肝细胞癌、胰腺癌研究中的进展[J]. 中华普通外科学文献(电子版), 2008, 2(3): 231-234. Wang H, Li W, Wang Q. Development of CD133 in hepatocellular and pancreatic carcinoma[J]. Chin Arch Gen Surg (Electron Vers), 2008, 2(3): 231-234. DOI:10.3969/j.issn.1674-0793.2008.03.017 |
| [22] |
杨阳. 趋化因子CXCL16及其受体CXCR6维持CD133+卵巢癌干细胞侵袭迁移能力的研究[D]. 重庆: 第三军医大学, 2015. Yang Y. Chemokine CXCL16 and its receptor CXCR6 maintain the invasion and migration of CD133+ ovarian cancer stem cells[D]. Chongqing: Third Mil Med Univ, 2015. http://cdmd.cnki.com.cn/Article/CDMD-90031-1015631963.htm |
 2018, Vol. 38
2018, Vol. 38


