2. 250117 济南, 山东大学附属山东省肿瘤医院放疗科
2. Department of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Jinan 250117, China
目前全乳放疗(whole breast irradiation,WBI)仍为早期乳腺癌保乳术后主流放疗方式,但对符合低复发风险特征的患者,部分乳腺照射(partial breast irradiation, PBI)替代WBI的可行性已被证实[1-2]。因此,PBI已成为早期低复发风险乳腺癌患者可选的放疗方式[3-5],而部分乳腺外照射(external-beam partial breast irradiation,EB-PBI)则是PBI的主要实现方式之一。尽管多数研究显示EB-PBI的疗效及其不良反应均可与WBI媲美,但也有研究显示EB-PBI的晚期不良反应发生率显著高于WBI,乳房美容效果优良率显著低于WBI[6]。无论是疗效保障,还是不良反应的降低,合理的靶区确定都是关键。因此,EB-PBI靶区确定目前仍然广受关注。EB-PBI靶区确定的影响因素众多,术后EB-PBI的影响因素包括切除标本体积、金属夹个数、定位影像类别、勾画者差异等[7],不同体位下实施EB-PBI时靶区确定也存在差异[8]。术前EB-PBI则是缩小靶区的有效途径,无论是肿瘤靶区(gross tumor volume,GTV)还是计划靶区(planning target volume,PTV)均显著缩小[9-10]。本研究拟基于形变配准比较保乳术前俯卧位诊断MRI与术后俯卧位定位CT瘤床靶区间体积和位置的关联与差异,明确基于术前俯卧位MRI辅助术后俯卧位EB-PBI靶区确定的获益程度,并明确术前MRI肿瘤靶区与术后俯卧位定位CT瘤床靶区间的关联性,从而为如何利用术前诊断MRI指导术后俯卧位EB-PBI靶区勾画提供参考。
资料与方法1.入组条件:早期乳腺癌保乳术后符合EB-PBI条件的患者;术前行俯卧位诊断MRI扫描;原发肿瘤切除方式为局部肿瘤扩大切除且无术腔塑形填充;术腔边界标记金属夹≥5个;自愿接受俯卧位CT扫描并签署知情同意书;放疗定位时术腔中无可见血清肿。
2.临床资料:2016年7月至2017年4月入住山东省肿瘤医院放疗科并行俯卧位EB-PBI的保乳术后早期乳腺癌患者30例,符合入组条件者18例。原发肿瘤位于左乳腺者7例,右乳腺者11例,外上象限11例,外下象限1例,内下象限4例,内上象限2例。全部患者原发肿瘤手术方式均为局部肿瘤扩大切除术,即基于手术医生触诊范围外扩1 cm的边界进行切除[11]。腋窝处置方式为前哨淋巴结活检或前哨淋巴结活检+腋窝淋巴结清扫。术后分期均为pT1N0M0。
3.图像采集:①术前诊断MRI扫描:患者俯卧位于MRI乳腺专用线圈上,头部面朝下置于专用头枕,双乳自然下垂,双手自然前伸置于头部两侧,行诊断扫描并获得T2WI等多序列图像。②术后俯卧位CT模拟定位:患者俯卧于俯卧位专用乳腺托架上,头部面朝下置于专用头枕上,双手上举握住手柄,患侧乳腺悬垂于治疗孔中,调整健侧挡板位置,使健侧乳腺尽可能远离患侧。CT扫描的范围从环甲膜至肺下缘下5 cm,层厚3 mm。将术前诊断MRI扫描及术后俯卧位CT模拟定位图像均传输至MIM图像处理系统。
4.靶区勾画和定义:由同一名医生分别在MIM图像处理系统中的术前诊断MRI图像及术后定位CT图像上进行靶区勾画与构建。在MRI图像T2WI序列勾画原发肿瘤并定义为GTVMRI,GTVMRI边界外扩10和20 mm所构建的临床靶区(clinical target volume,CTV)分别定义为CTVMRI+1和CTVMRI+2,GTVMRI边界外扩15和25 mm所构建的计划靶区(planning target volume)分别被定义为PTVMRI+1和PTVMRI+2,勾画患侧全乳靶区CTVBreast-MRI;在俯卧位CT定位扫描图像上,基于术腔金属夹勾画瘤床(tumor bed, TB)靶区并定义为GTVTB,边界外扩10和15 mm分别定义为CTVTB和PTVTB。勾画患侧全乳靶区CTVBreast-CT(图 1)。
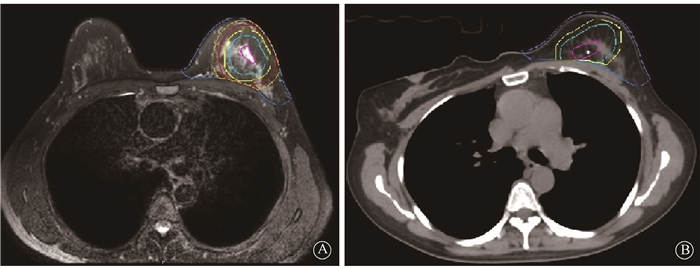
|
图 1 基于术前俯卧位诊断MRI及术后俯卧位定位CT所勾画的靶区图 A.术前诊断MRI;B.术后定位CT Figure 1 The picture of target volumes based on preoperative prone diagnostic MRI or postoperative prone simulation CT A. Preoperative diagnostic MRI; B. Postoperative simulation CT |
5.图像形变配准:应用MIM图像处理系统的形变配准软件进行形变配准。首先选定主要序列及从属序列进行刚性配准,然后在此基础上实现自动形变配准。在本研究的配准过程中,均以俯卧位CT定位图像作为主要序列,以MRI的T2WI图像作为从属序列。在完成自动形变配准后,应用Reg Reveal和Reg Refine工具对形变配准后的图像进行配准质量的评估及修正,以实现视觉上最佳的配准效果。
6.统计学处理:采用SPSS 19.0软件对数据进行处理,不服从正态分布的计量资料采用秩和检验。靶区体积、相关性检验以及相关参数采用Spearman分析。P < 0.05为差异有统计学意义。
结果1.靶区体积比较及相关性分析:基于MRI及CT图像定义的各靶区体积列于表 1。GTVTB较GTVMRI大12.58 cm3(Z=-3.593,P<0.05),CTVMRI+1和PTVMRI+1分别小于CTVTB和PTVTB(Z=-3.593,-2.983,P<0.05),而CTVMRI+2和PTVMRI+2则依次大于CTVTB和PTVTB(Z=-2.722,-2.853, P<0.05)。GTVMRI与GTVTB、CTVMRI+1与CTVTB、CTVMRI+2与CTVTB间均呈正相关(r=0.518, 0.474,0.498,P<0.05),而PTVMRI+1与PTVTB间及PTVMRI+2与PTVTB间则均无相关性(r=0.401, 0.377,均P>0.05),见表 2。
|
|
表 1 18例乳腺癌患者术前俯卧位MRI及术后俯卧位定位CT所勾画与构建靶区体积(cm3) Table 1 Target volume defined using preoperative prone MRI and postoperative prone CT(cm3) |
|
|
表 2 18例乳腺癌患者术前俯卧位MRI及术后俯卧位定位CT所勾画靶区体积的相关性 Table 2 Correlation between the target volumes defined using preoperative prone MRI and postoperative prone CT |
2.基于形变配准的MRI与CT靶区间空间位置比较:基于胸廓形变配准时,CTVBreast-MRI与CTVBreast-CT之间适形指数(conformity index,CI)、包含度(degree of inclusion,DI)、相似度指数(dice similarity coefficient, DSC)分别为0.56、0.82、0.71,靶区中心距离(ΔV)为1.81 cm。基于胸廓形变配准时,GTVTB-GTVMRI、GTVTB-CTVMRI+1、CTVTB-GTVMRI、CTVTB-CTVMRI+1之间CI值均较小,分别为0.02、0.07、0.04、0.17,基于GTVMRI与GTVTB靶区中心点进行配准时,对应CI值虽显著增加,但仍然较小,依次为0.19、0.31、0.05、0.38。MRI与CT对应靶区间DI及DSC值均较小,但基于胸廓配准与基于靶区中心配准所对应的DI间及DSC间差异有统计学意义的(Z=-3.724、-3.724、-3.201、-3.724,和-3.724、-3.724、-2.591、-3.636,P < 0.05)(表 3)。
|
|
表 3 18例乳腺癌患者胸廓形变配准与靶区中心配准时MRI与CT对应靶区间相关参数比较 Table 3 Evaluation of the parametersof the target volume defined using preoperative prone MRI and postoperative prone CT based on DIR of the thorax or target center |
讨论
近年来,术后PBI替代WBI研究的远期结果陆续发布,总体上看,只要病例选择恰当,无论是疗效还是美容效果,PBI均可媲美WBI,但目前多数指南和共识仍没有将PBI作为早期乳腺癌保乳术后放疗的常规方法推荐,主要原因是相关研究结果有待远期随访,而且部分研究结果提示应警示PBI的不良反应,如Olivotto等[6]研究中期结果显示,基于三维适形放疗(3DCRT)实施EB-PBI时,无论是护士评估还是患者自身评估,EB-PBI组患者美容效果差评率均显著高于WBI组,分别为29%和17%、26%和18%(P < 0.001),EB-PBI组患者1~2度不良反应的发生率也显著高于WBI组(P < 0.001)。上述研究的中期结果促使研究者去反思术后EB-PBI的各方面,首要的是术后EB-PBI靶区确定。实际上,保乳术后EB-PBI靶区确定是一个复杂的过程,瘤床靶区的确定受到了术者因素、术式因素、术腔因素及勾画者因素等多方面的影响[7]。
毋庸置疑,EB-PBI靶区的缩小会相应降低正常乳腺受照剂量,从而为减轻不良反应和保障美容效果提供条件[12]。术前EB-PBI替代术后EB-PBI也是缩小靶区的途径。已有研究报道,术前EB-PBI与术后EB-PBI相比,无论是GTV还是PTV均显著缩小[9-10]。本研究在术前俯卧位诊断MRI和术后俯卧位CT图像上分别基于原发肿瘤和术腔金属夹进行GTV勾画,结果显示,基于术后瘤床勾画的GTVTB显著大于基于术前原发肿瘤勾画的GTVMRI,这一结果与文献[9]报道相同。本研究显示,基于GTVMRI外扩10和15 mm构建的CTVMRI+1和PTVMRI+1分别小于基于GTVTB外扩10和15 mm构建的CTVTB和PTVTB,但基于GTVMRI外扩20和25 mm构建的CTVMRI+2和PTVMRI+2则分别大于CTVTB和PTVTB,从本研究的这一结果看,如果外扩范围过大,术前EB-PBI就会失去正常乳腺照射减少的优势,而外扩范围的主要参考因素为亚临床灶范围。10 mm是保乳治疗时局部肿瘤扩大切除所普遍采用的外扩边界,也是早期研究所报道的亚临床灶范围[13-14],因此,基于GTVMRI外扩10 mm构建的CTVMRI是合理的。但也有研究显示,基于MRI引导保乳肿瘤切除时,52%的标本在GTVMRI外扩10 mm后仍可发现亚临床病灶,25%的标本在GTVMRI外扩20 mm后仍可见亚临床病灶[15],这可能是术前加速部分乳腺癌外照射(APBI)研究选择基于GTVMRI外扩20和25 mm构建CTVMRI和PTVMRI的参考。本研究没有比较基于GTVMRI外扩15 mm构建CTVMRI与基于术后瘤床构建CTVTB的大小,但文献报道两者差异无统计学意义[9]。
理论上讲,如果等边界切除肿瘤,则基于术前MRI可见肿瘤与基于术后瘤床等边界外扩构建的CTVMRI和CTVTB体积差异应当是不显著的,而造成两者差异的主要原因是肿瘤的非对称性切除[9],如果在基于术腔勾画和构建靶区时考虑到因非对称性切除所致边界的各向异性,CTVMRI和CTVTB体积差异则并不显著[8]。通常情况下,医生基于触诊范围外扩切除边界,从而导致非对称性切除,表现为术前MRI可见肿瘤体积与切除标本体积及瘤床体积间并无相关性[16-17]。本研究显示,术后GTVTB与术前GTVMRI以及CTVMRI+1均呈正相关,可能的原因是近年来在进行保乳手术局部肿瘤切除时,除了依据触诊进行切除范围的确定外,越来越注重对术前MRI等影像资料的参考,从而减小了所切标本边缘距肿瘤边缘的各向异性。
MRI不仅是实施术前EB-PBI的基础,也有助于术后EB-PBI病例的筛选,并可指导术后EB-PBI的靶区勾画[18-19]。术前诊断性乳腺MRI图像是基于俯卧位获得的,患者体位与保乳术后俯卧位放疗体位相同,理论上讲,术前诊断性MRI图像与术后俯卧位CT图像形变配准应当有助于术后俯卧位EB-PBI靶区确定,但本研究结果看,无论是术前GTVMRI与术后GTVTB之间,还是GTVMRI+1与GTVTB之间,基于胸廓形变配准后靶区间CI、DI、DSC均较差,且两靶区中心点的距离长达2.71和2.67 cm。本研究显示,[JP+1]术前诊断性MRI与术后俯卧位定位CT两种图像间全乳空间位置匹配并不理想,表现为CTVBreast-MRI与CTVBreast-CT间基于胸廓形变配准后CI、DI、DSC均未达到1。造成全乳不匹配的主要原因可能是,术前行俯卧位MRI扫描所采用的线圈可使患者双乳自然下垂,术后俯卧位定位CT采用的托架则会使患者被治疗的乳腺悬垂于治疗孔,而健侧乳腺被挡板牵拉远离患侧腺体,这在一定程度上影响了患侧腺体的自然悬垂状态。全乳匹配不理想是导致GTVMRI及GTVMRI+1与GTVTB间空间错位明显的主要原因之一。当然另一个主要原因则在于GTVMRI及GTVMRI+1与GTVTB间空间形态是不一样的,从本研究结果看,即便是基于靶区中心点配准,GTVMRI与GTVTB间、GTVMRI+1与GTVTB间的CI、DI、DSC仍然不理想,但较基于胸廓配准有显著改善。
综上所述,对基于俯卧位实施EB-PBI的早期乳腺癌患者而言,基于术前俯卧位MRI所得靶区体积显著小于基于术后俯卧位CT所得靶区体积,且对应靶区之间呈正相关。即便进行形变配准,术前俯卧位诊断MRI与术后俯卧位定位CT间,无论全乳靶区间还是肿瘤靶区与瘤床靶区间的空间对应性仍然较差,因此,基于术前诊断MRI与术后瘤床进行俯卧位EB-PBI靶区的融合性勾画是不合理。
利益冲突 本文作者与单位没有因此项研究工作接受过第三方的资助或服务。不存在与本工作职责相冲突的经济利益作者贡献声明 于婷负责研究的具体实施和论文撰写;李建彬与王玮参与论文选题与设计;徐敏、邵倩、张英杰、刘希军参与病例入组及资料的收集与整理
| [1] |
Polgár C, Fodor J, Major T, et al. Breast-conserving therapy with partial or whole breast irradiation:ten-year results of the Budapest randomized trial[J]. Radiother Oncol, 2013, 108(2): 197-202. DOI:10.1016/j.radonc.2013.05.008 |
| [2] |
Polgár C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast:5-year results of a randomised, controlled, phase 3 trial[J]. Lancet Oncol, 2017, 18(2): 259-268. DOI:10.1016/S1470-2045(17)30011-6 |
| [3] |
Polgár C, van Limbergen E, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery:recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009)[J]. Radiother Oncol, 2010, 94(3): 264-273. DOI:10.1016/j.radonc.2010.01.014 |
| [4] |
Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO)[J]. J Am Coll Surg, 2009, 209(2): 269-277. DOI:10.1016/j.jamcollsurg.2009.02.066 |
| [5] |
Shah C, Vicini F, Shaitelman SF, et al. The American Brachytherapy Society consensus statement for accelerated partial-breast irradiation[J]. Brachytherapy, 2018, 17(1): 154-170. DOI:10.1016/j.brachy.2017.09.004 |
| [6] |
Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID:a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy[J]. J Clin Oncol, 2013, 31(32): 4038-4045. DOI:10.1200/JCO.2013.50.5511 |
| [7] |
van Mourik AM, Elkhuizen PH, Minkema D, et al. Multiinstitutional study on target volume delineation variation in breast radiotherapy in the presence of guidelines[J]. Radiother Oncol, 2010, 94(3): 286-291. DOI:10.1016/j.radonc.2010.01.009 |
| [8] |
于婷, 王玮, 徐敏, 等. 保乳术后仰卧位与俯卧位部分乳腺外照射靶区体积及剂量学比较[J]. 中华放射医学与防护杂志, 2018, 38(2): 93-99. Yu T, Wang W, Xu M, et al. A comparison of the target volume and dosimetric variance between supine and prone positions for external-beam partial breast irradiation after breast-conserving surgery[J]. Chin J Radiol Med Prot, 2018, 38(2): 93-99. DOI:10.3760/cma.j.issn.0254-5098.2018.02.003 |
| [9] |
van der Leij F, Elkhuizen PH, Janssen TM, et al. Target volume delineation in external beam partial breast irradiation:less inter-observer variation with preoperative-compared to postoperative delineation[J]. Radiother Oncol, 2014, 110(3): 467-470. DOI:10.1016/j.radonc.2013.10.033 |
| [10] |
Nichols EM, Dhople AA, Mohiuddin MM, et al. Comparative analysis of the post-lumpectomy target volume versus the use of pre-lumpectomy tumor volume for early-stage breast cancer:implications for the future[J]. Int J Radiat Oncol Biol Phys, 2010, 77(1): 197-202. DOI:10.1016/j.ijrobp.2009.04.063 |
| [11] |
Rutgers EJ. Quality control in the locoregional treatment of breast cancer[J]. Eur J Cancer, 2001, 37(4): 447-453. DOI:10.1016/S0959-8049(00)00386-5 |
| [12] |
Palta M, Yoo S, Adamson JD, et al. Preoperative single fraction partial breast radiotherapy for early-stage breast cancer[J]. Int J Radiat Oncol Biol Phys, 2012, 82(1): 37-42. DOI:10.1016/j.ijrobp.2010.09.041 |
| [13] |
Faverly DR, Burgers L, Bult P, et al. Three dimensional imaging of mammary ductal carcinoma in situ:clinical implications[J]. Semin Diagn Pathol, 1994, 11(3): 193-198. |
| [14] |
Clark RM, Wilkinson RH, Miceli PN, et al. Breast cancer. Experiences with conservation therapy[J]. Am J Clin Oncol, 1987, 10(6): 461-468. DOI:10.1097/00000421-198712000-00001 |
| [15] |
Schmitz AC, van den Bosch MA, Loo CE, et al. Precise correlation between MRI and histopathology-exploring treatment margins for MRI-guided localized breast cancer therapy[J]. Radiother Oncol, 2010, 97(2): 225-232. DOI:10.1016/j.radonc.2010.07.025 |
| [16] |
张爱苹, 李建彬, 王玮, 等. 保乳术前肿瘤MRI体积与术后标本体积及基于金属夹勾画术腔体积比较研究[J]. 中华放射肿瘤学杂志, 2016, 25(12): 1314-1318. Zhang AP, Li JB, Wang W, et al. Gross tumor volume delineation in patients undergoing breast-conserving surgery:a comparative study of preoperative magnetic resonance imaging, postoperative specimen, and lumpectomy cavity based on surgical clips[J]. Chin J Radiat Oncol, 2016, 25(12): 1314-1318. DOI:10.3760/cma.j.issn.1004-4221.2016.12.009 |
| [17] |
den Hartogh MD, van Asselen B, Monninkhof EM, et al. Excised and irradiated volumes in relation to the tumor size in breast-conserving therapy[J]. Breast Cancer Res Treat, 2011, 129(3): 857-865. DOI:10.1007/s10549-011-1696-7 |
| [18] |
Pengel KE, Loo CE, Teertstra HJ, et al. The impact of preoperative MRI on breast-conserving surgery of invasive cancer:a comparative cohort study[J]. Breast Cancer Res Treat, 2009, 116(1): 161-169. DOI:10.1007/s10549-008-0182-3 |
| [19] |
Tendulkar RD, Chellman-Jeffers M, Rybicki LA, et al. Preoperative breast magnetic resonance imaging in early breast cancer:implications for partial breast irradiation[J]. Cancer, 2009, 115(8): 1621-1630. DOI:10.1002/cncr.24172 |
 2018, Vol. 38
2018, Vol. 38


