2. State Key Laboratory of Trauma, Burns and Combined Injury, Third Military Medical University, Chongqing 400038, China
Liver is one of the most important organs. Healthy hepatic cells exhibit many biologic functions, such as metabolism of nutrients, synthesis of proteins, production of bile, excretion of toxicant, etc. However, hepatic cells are also quite sensitive to many harmful factors. The large dose of total body irradiation (TBI) usually caused severe damages in hemotapoietic tissues and gastrointestinal tract. However, its influence on hepatic tissues has not been fully clarified. In addition, radiotherapy for hepatocellular carcinoma (HCC) frequently induced radiation induced liver disease (RILD), which significantly restricted the application of radiotherapy in the treatment of HCC[1-3]. Therefore, understanding the pathological changes of hepatic cells after ionizing radiation (IR) is required to improve the better treatment of RILD.
Autophagy degrades intracellular components to sustain metabolism and homeostasis when required, and protects cells from injury by preventing the accumulation of damaged proteins and organelles in cells [4-6]. IR can induce autophagy and damage in normal and cancer cells. Various studies have shown that autophagy plays an important role in radiation-induced tissue damage[7-9]. In the liver, autophagy has been shown to be associated with the physiology and pathogenesis[10-11], including liver steatosis, partial hepatectomy, and nonalcoholic fatty liver conditions[12-14]. However, whether and how autophagy participates the response of hepatic cells after irradiation has not been well explored.
In this study, the protective effects of autophagy for hepatic cells in the IR-induced liver injury and the potential mechanisms were investigated.
Materials and methods 1. MiceAdult C57BL/6 mice (6-8 weeks old, weighing 20-25 g) were provided by Experimental Animal Center, Third Military Medical University(SCXK2017-0002). GFP-LC3 transgenic mice were genotyped[15] and kept in the SPF facility. All the experimental protocols in this study were approved by the Ethics Committee and were conducted in accordance with the Animal Care and Use Committee Guidelines of the Third Military Medical University.
2. Chemicals and ReagentsCell culture media and supplements were from Hyclone (Shanghai, China). LC3 antibody was from Cell Signaling Technology (Shanghai, China). Mouse monoclonal antibody against 8-Hydroxy-2′deoxy Guanosine (8-OHdG) was from Abcam (Shanghai, China). GFP, PARP and Caspase-3 antibodies were from Beyotime (Hangzhou, China). Chloroquine (CQ), Rapamycin (Rap) and N-acetyl-cysteine (NAC) were purchased from Sigma-Aldrich(USA). CQ and Rapamycin were dissolved in dimethyl sulfoxide (DMSO) and diluted with culture media before use. NAC was dissolved in culture media. The final concentration of DMSO in all cultures was 0.01%.
3. Cell cultureL02 cells were grown in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin. Cells were maintained at 37℃ in a humidified incubator with 5% CO2.
4. Radiation and treatmentsMice were divided into 6 groups and received total body irradiation with a dose of 8 Gy at 0.985 Gy/min[16]. L02 cells were irradiated at 12 Gy by an X-RAD 320 Biological Irradiator. In order to explore the role of autophagy in radiation-induced liver injury, autophagy inhibitor chloroquine (60 mg/kg) and inducer rapamycin (2 mg/kg) were injected by intraperitoneal administration 2 h prior to irradiation.
5. Measurement of liver functionMice were anaesthetized with 1% barbital sodium at 12 h after IR. Blood was collected via cardiac puncture, and the serum was separated with standard protocol for the liver function assay. The alanine aminotransferase (ALT), aspertate aminotransferase (AST) levels in serum were tested with an Automated Chemical Analyzer in the Southwest Hospital (Chongqing, China) according to the manufacturer′s instructions.
6. Histological and TUNEL stainingLiver tissues were fixed in 4% paraformaldehyde for 24 h, and dehydrated and embedded regularly. Tissue sections with 4 μm thickness were used for staining. Briefly, deparaffinized slides were stained with Meyer′s hematoxylin and eosin (H & E). In situ apoptosis was stained with TUNEL kit (Roche, Germany) to assess the apoptosis level in liver tissues according to the manufacturer′s instructions.
7. ImmunostainingTo examine autophagic puncta in hepatic cells, GFP antibody (1:200) was used to stain GFP-LC3 liver tissues by immunohistochemical staining. In brief, deparaffinized slides were blocked and incubated with primary antibody overnight at 4℃. After washing and incubation with HRP conjugated secondary antibody for 1 h at room temperature, DAB was applied for 30 s and then washed off in distilled water. Finally, slides were counterstained with hematoxylin. DNA oxidative damage was stained by immunofluorescence of 8-OHdG antibody (1:200) in liver of mice after irradiation as previously described[16]. Images were taken with Olympus BX51 microscope.
8. Western blot analysisTotal proteins from L02 cells or liver tissues were analyzed by Western blot. The proteins were extracted and resolved by SDS-PAGE, and proteins were electroblotted onto PVDF membrane, then blocked with 5% fat-free milk at room temperature for 1 h. Membranes were incubated with diluted primary antibodies (1:1 000) overnight at 4℃. After incubation with secondary antibody for 1 h at room temperature, the intensity of bands was visualized and determined by enhanced chemiluminescence (ECL) detection system (Bio-Rad, USA)
9. Flow cytometric analysisApoptosis was detected using Annexin-V reagents (Beyotime, China) following the provided operating manual. Briefly, cells were collected and resuspended in 200 μl 1× binding buffer and incubated with 5 μl of Annexin V and 5 μl of propidium iodide(PI) for 15 min at room temperature in the dark. After incubation, samples were analyzed by BD Accuri C6 flow cytometer within 1 h. Results were analyzed by FlowJo software.
10. Cell proliferation assayL02 cells were seeded into 96-well plates (4×103 cells per well) and then pretreated with 10 mmol/L NAC for 2 h. Next, cells were exposed to 12 Gy X-ray radiation only or incubated with CQ (10 μmol/L) for 24 h. Cell proliferation was assessed by CCK-8 (Dojindo) reagents flowing manufacture′s instruction. After 2 h of incubation at 37℃, the absorbance at 450 nm was recorded using a microplate reader (Thermo Scientific, USA).
11. Determination of ROS generationThe levels of ROS was detected by 2′, 7′-dichloro-cluorescein diacetate (DCFH-DA, Beyotime, China). Cells were collected and washed twice with PBS, and then incubated in serum-free media with 10 μmol/L DCFH-DA for 20 min at 37℃ in the dark. After three washes in PBS, cells were imaged by Olympus BX51 fluorescence microscopy.
12. Statistical analysisAll data were expressed as x±s. The difference between two groups was determined with the Student′s t test, and was considered to be statistically significantly different when P value was less than 0.05.
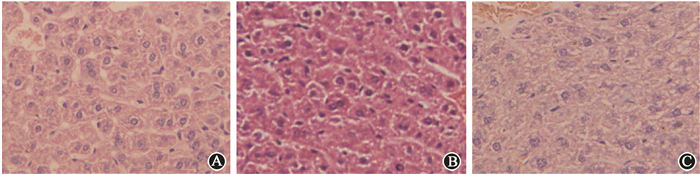
Results 1. Irradiation resulted in hepatic injuryThe levels of AST were significantly elevated in the IR group compared with the control group at 12 h after irradiation, but there was no statistically significant difference at ALT levels between these two groups (Table 1). Hepatic cells in IR group had a misarranged morphology, and some nuclei were shrunk at 24 h (Figure 1). Many TUNEL positive cells appeared in the irradiated liver tissues (Figure 2), hardly to be seen in the control group. Western blots demonstrated markedly increased level of cleaved Caspase-3 and cleaved PARP, which were representative markers for apoptosis, indicating that IR could induce obvious apoptosis of hepatic cells. Meanwhile, γ-H2AX expression was obviously increased after radiation in liver tissue (Figure 3). These data demonstrated that IR administration definitely caused liver injury.
|
Figure 1 Representative H & E images of liver tissues ×800 A. Control; B. 12 h after irradiation; C. 24 h after irradiation |

|
Figure 2 In situ apoptosis was detected by TUNEL kit (apoptotic cells were indicated by arrows)×400 A. Control; B. 12 h after irradiation; C. 24 h after irradiation |
|
Figure 3 γ-H2AX expression obviously increased after irradiation ×400 A. Control; B. 12 h after irradiation; C. 24 h after irradiation |
|
|
Table 1 The levels of ALT and AST in mouse serum after irradiation(x±s) |
2. Elevation of autophagy in mouse liver after irradiation
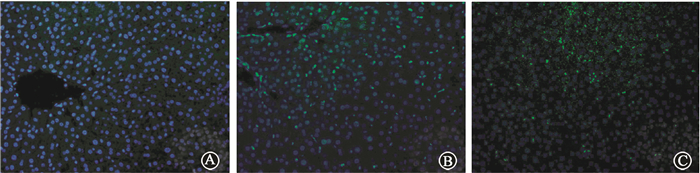
GFP-LC3 mice were terminated at 12 h and 24 h after irradiation. More GFP positive autophagic dots were found in IR groups compared with the control group (Figure 4). Meanwhile, the protein levels of LC3 and p62 were examined, which were two characteristic markers of autophagosomal activation. As shown in Figure 5, LC3 levels were significantly increased in IR group, while p62 levels were decreased. These results suggested that irradiation could activate autophagy flux in mouse liver.
|
Figure 4 Immunohistochemical staining demonstrated more GFP positive LC3 puncta in the liver of mice after irradiation ×1 000 A. Control; B. 12 h after irradiation; C. 24 h after irradiation |
|
Figure 5 The levels of LC3 and p62 after irradiation detected by Western blot Note: 1. Control; 2. 12 h after irradiation; 3. 24 h after irradiation |
3. Manipulation of autophagy affected IR induced hepatic injury in vivo
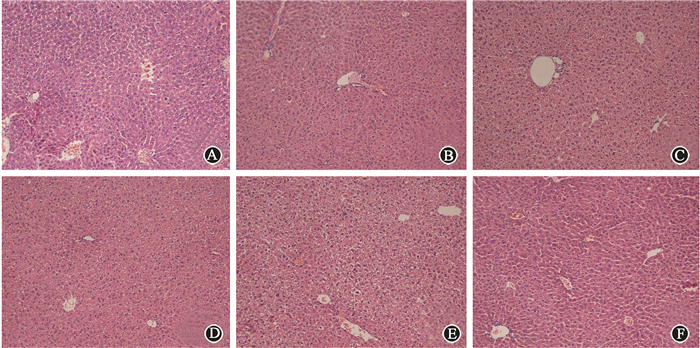
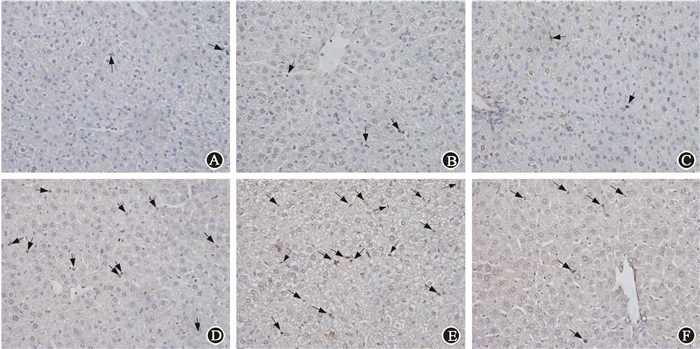
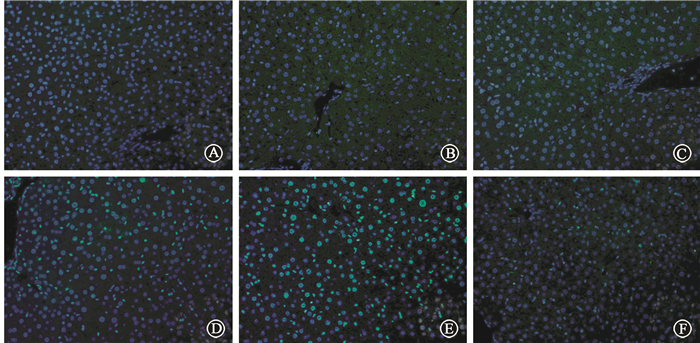
Mice were pretreated with CQ or Rap respectively 2 h before irradiation. None of CQ, Rap or DMSO would influence the levels of AST and ALT in serum. Pretreatment with CQ significantly elevated the serum AST level compared with irradiation alone. Unexpectedly, CQ also significantly increased the ALT level at 12 h, which was not changed when mice were only treated by IR. Rapamycin partly decreased IR-induced higher AST level, and had no influence on ALT lelvel(Table 2). Mice pretreated with CQ had worse morphological alterations compared with those only with irradiation, including unclear structure of hepatic lobules, disarranged hepatocyte cords, narrowed hepatic sinusoids and swollen hepatocytes. Pretreatment with rapamycin effectively reversed those changes (Figure 6). Pretreatment with CQ resulted in higher apoptosis of hepatic cells. However, mice pretreated with rapamycin had less apoptotic cells (Figure 7). Meanwhile, γ-H2AX expression was obviously increased after irradiation in liver tissue. Stronger intensity of γ-H2AX was found when mice were administered with the combination of irradiation and CQ, while rapamycin decreased its expression(Figure 8). These results suggested that pretreatment with CQ greatly exacerbated radiation-induced liver injury, and rapamycin reversed hepatic injury.
|
Figure 6 Representative H & E images of CQ or rapamycin treated livers ×200 A. Control; B. CQ; C. Rap; D. IR; E. IR+CQ; F. IR+Rap |
|
Figure 7 Pretreated with CQ had more TUNEL positive apoptotic cells (indicated by arrows) in liver after irradiation, and rapamycin treated mice had less apoptotic cells ×400 A. Control; B. CQ; C. Rap; D. IR; E. IR+CQ; F. IR+Rap |
|
Figure 8 Immunofluorescent staining of γ-H2AX ×400 A. Control; B. CQ; C. Rap; D. IR; E. IR+CQ; F. IR+Rap |
|
|
Table 2 The levels of ALT and AST of each group(x±s) |
4. Activation of autophagy reduced IR-induced apoptosis in vitro
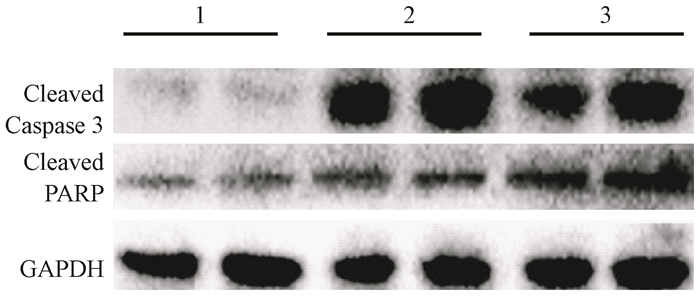
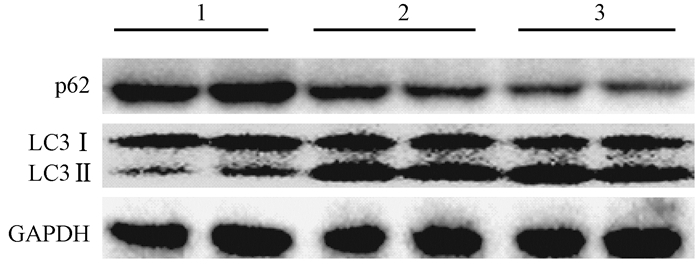
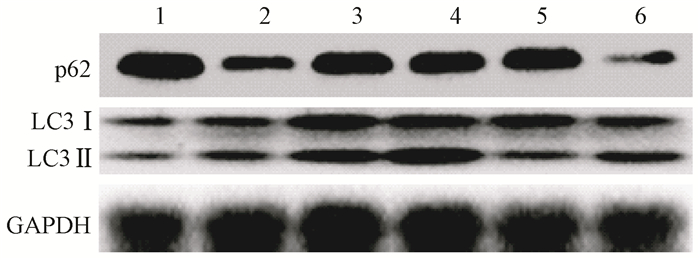
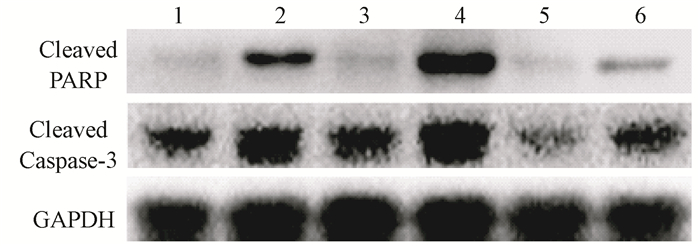
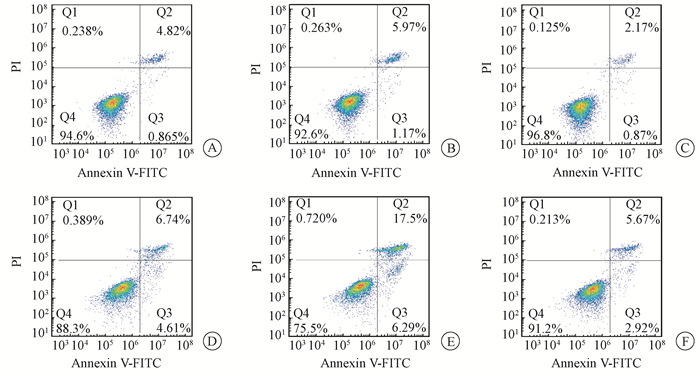
The levels of LC3 were significantly increased after irradiation while p62 level was decreased in L02 cells. Administration with CQ after irradiation further increased the levels of LC3 and retarded p62 degradation. Meanwhile, administration with rapamycin significantly elevated the level of LC3 and increased p62 degradation (Figure 9). Furthermore, the levels of cleaved Caspase-3 and cleaved PARP were markedly increased in the IR groups. The levels of cleaved Caspase-3 and cleaved PARP in L02 cells treated with CQ were further increased. Similarly, rapamycin had partially alleviated apoptosis in L02 cells after IR(Figure 10).The percentage of apoptotic cells in the group, which received IR and CQ together, was significantly higher than that of IR alone(Figure 11). Meanwhile, rapamycin significantly reduced the percentage of apoptotic cells. Therefore, these results demonstrated that induction of autophagy protected the liver cells against radiation-induced apoptosis.
|
Figure 9 The levels of LC3 and p62 in each group were detected by western blot Note: 1. Control; 2. IR; 3. CQ; 4. IR+CQ; 5. Rap; 6. IR+Rap |
|
Figure 10 Pretreatment with CQ increased cleaved PARP and Caspase-3 levels, while rapamycin decreased apoptosis L02 cells Note: 1. Control; 2. IR; 3. CQ; 4. IR+CQ; 5. Rap; 6. IR+Rap |
|
Figure 11 Flow cytometry showed that CQ induced more apoptosis of L02 cells, and rapamycin decreased apoptosis of L02 cells A. Control; B. CQ; C. Rap; D. IR; E. IR+CQ; F. IR+Rap |
5. Autophagy protected IR damaged hepatic cells by decreasing excessive ROS
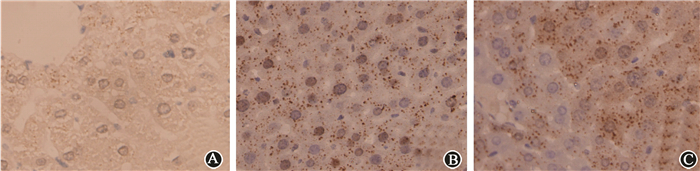
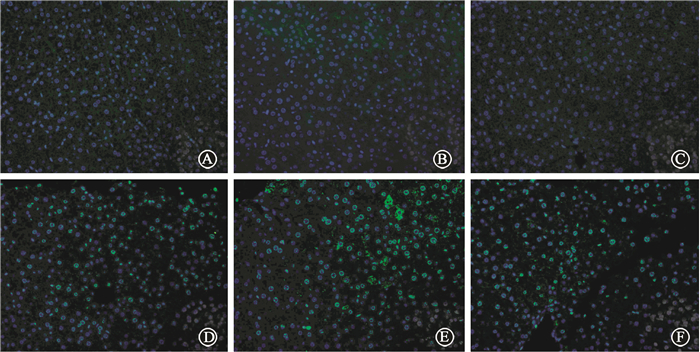
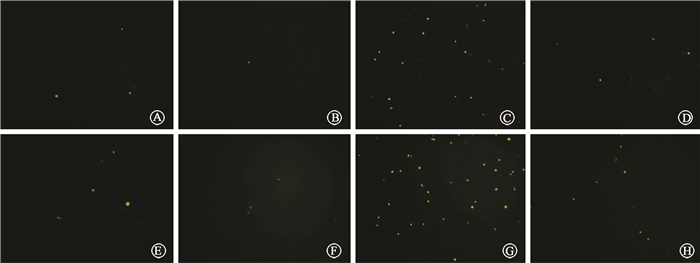
Excessive generation of ROS after radiation is one of the most important factors that cause tissue damage, and NAC can effectively remove ROS. We determine the role of NAC on radiation-induced liver injury. It was found that NAC prominently reduced the ALT and AST levels in mice treated with IR and CQ together (Table 3). The oxidative damage in liver tissue was also investigated by 8-OHdG, 8-OHdG expression was obviously increased after radiation in liver tissue. Stronger intensity of 8-OHdG was found when mice were treated with the combination of radiation and CQ, while rapamycin decreased its expression (Figure 12). ionizing radiation effectively increased the level of ROS in L02 cells, while CQ treatment markedly increased ROS. And NAC effectively decreased ROS levels in both radiation treated L02 cells and cells treated with IR and CQ (Figure 13). NAC also significantly alleviated the decreased cell viability caused by IR and CQ (Figure 14). These results suggested that ROS was involved in the IR induced hepatic toxicity, and eliminating excessive ROS was one of the potential mechanisms by which autophagy protected hepatocytes from IR-induced liver injury.
|
Figure 12 Immunofluorescent staining of 8-OHdG ×400 A. Control; B. CQ; C. Rap; D. IR; E. IR+CQ; F. IR+Rap |
|
Figure 13 Cellular ROS activity was examined in L02 cells using DCFH-DA kit ×200 A. Control; B. NAC; C. IR; D. IR+NAC; E. CQ; F. CQ+NAC; G. IR+CQ; H. IR+CQ+NAC |
|
Figure 14 Cell viability was examined in L02 cells using CCK-8 kit Note: 1. Control; 2. CQ; 3.NAC; 4.IR; 5.IR+NAC; 6.IR+CQ; 7.IR+CQ+NAC; aCompared with the IR group, t =2.87, P < 0.05; bCompared with the IR+ CQ group, t =6.398, P < 0.05 |
|
|
Table 3 The levels of ALT and AST of each group(x±s) |
Discussion
As an evolutionary conserved process, autophagy is usually considered as degradation of long-lived proteins and excess or dysfunctional organelles. Failure to activate autophagy under damaging conditions leads to cell death due to accumulation of damaged proteins and organelles. Autophagy has been proved to participate in many pathological processes in different context. Autophagy is upregulated in response to oxidative stresses such as ischemia/reperfusion steatosis, alcoholic consumption and toxic drugs. Autophagy lessens ischemic liver injury by reducing oxidative damage and activation of autophagy protects against cholestasis-induced hepatic injury [13, 17]. Meanwhile, autophagy over-activation could also cause self-destructive process and promoted the process of apoptosis and necrosis[18-19]. Therefore, autophagy is a double-edge sword for cells exposed to stressful conditions, and the specific role of autophagy depends on specific biological process.
Radiation results in DNA strandbreaks that lead to mutagenesis, apoptosis, senescence, or mitotic catastrophe[20]. In the present study, we found that 8 Gy ionizing radiation definitely caused hepatic injury as previously described [21-22], although ALT was not changed as quickly as AST, which might be due to early examination. Meanwhile, autophagy was significantly increased after ionizing radiation. To explore the role of autophagy in radiation-induced liver injury, autophagy inhibitor CQ and inducer rapamycin were used to manipulate the level of autophagy. Blockade of autophagy with CQ aggravated IR-induced hepatic injury and apoptosis of L02 cells. On the contrary, rapamycin partly reduced IR-induced apoptosis via activating autophagy. Therefore, this study speculates that increased autophagy after radiation is a self-protection mechanism of liver cells, autophagy is a critical mechanism of hepatic cells in response to irradiation. This study showed that the autophagic level in vivo was more evident than in vitro. Because the liver is a highly radiosensitive organ, hepatocytes are considered more radioresistant than other cells, which might be a reason for the difference between in vivo and in vitro[23].
Major ROS was generated by the mitochondrial respiratory chain, which acted as signaling molecules to regulate a variety of cellular functions, such as cell survival and senescence[24]. Normally, ROS was scavenged by complicate cellular antioxidant mechanisms and kept in balance. While ROS generation goes beyond the cellular antioxidant capacity, excessive ROS activates stress-activated signaling cascades and oxidative stress damage occurs [25-26]. ROS level was significantly increased after ionizing radiation, which resulted in cell death and liver damage. When autophagy was inhibited by CQ, increased ROS could be detected. However, rapamycin and NAC markedly reduced ROS accumulation. These findings indicate that ROS contributes to IR-induced liver injury and regulation of ROS, which is the potential mechanism that autophagy alleviates hepatic injury. Since mitophagy is the elimination of an important source of ROS[27], it is conceivable that autophagic degradation of damaged mitochondria in hepatic cells might be a part of the protection mechanism against radiation induced liver injury.
In conclusion, this study showed that enhancing autophagy could alleviate IR-induced hepatic injury. On the basis of these results, properly promoting autophagy can reduce the radiation-induced detrimental effects in the liver, which might be a potential target to optimize a possible therapeutic strategy for the radiotherapy of HCC and other cancers.
Conflict of interest statement The authors declare that there is no conflict of interestsContribution statement of authors Liu Lang and Liu Dengqun designed and carried out experiments, analyzed data and drafted the manuscript; Wang Yu and Wang Ziwen carried out experiments and analyzed data; Chen Zelin, Chen Jie and Han Xiao analyzed data; Liu Zujuan carried out experiments; Zhang Aihua and Shi Chunmeng designed and supevised this study
| [1] | Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury[J]. Int J Radiat Oncol Biol Phys, 2010, 76 (3 Suppl): S94-100. DOI:10.1016/j.ijrobp.2009.06.092. |
| [2] | Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives[J]. Exp Mol Med, 2017, 49 (7): e359 DOI:10.1038/emm.2017.85. |
| [3] | Cheema AK, Pathak R, Zandkarimi F, et al. Liver metabolomics reveals increased oxidative stress and fibrogenic potential in gfrp transgenic mice in response to ionizing radiation[J]. J Proteome Res, 2014, 13 (6): 3065-3074. DOI:10.1021/pr500278t. |
| [4] | Gunst J, Derese I, Aertgeerts A, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness[J]. Crit Care Med, 2013, 41 (1): 182-194. DOI:10.1097/CCM.0b013e3182676657. |
| [5] | Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade[J]. Nat Rev Mol Cell Biol, 2007, 8 (11): 931-937. DOI:10.1038/nrm2245. |
| [6] | Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy[J]. Curr Opin Cell Biol, 2010, 22 (2): 206-211. DOI:10.1016/j.ceb.2009.12.002. |
| [7] | Xu F, Li X, Yan L, et al. Autophagy promotes the repair of radiation-induced DNA damage in bone marrow hematopoietic cells via enhanced STAT3 signaling[J]. Radiat Res, 2017, 187 (3): 382-396. DOI:10.1667/RR14640.1. |
| [8] | Liu Q, Sun Y, Lv Y, et al. TERT alleviates irradiation-induced late rectal injury by reducing hypoxia-induced ROS levels through the activation of NF-kappaB and autophagy[J]. Int J Mol Med, 2016, 38 (3): 785-793. DOI:10.3892/ijmm.2016.2673. |
| [9] | Hou J, Han ZP, Jing YY, et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells[J]. Cell Death Dis, 2013, 4 : e844 DOI:10.1038/cddis.2013.338. |
| [10] | Arroyo DS, Gaviglio EA, Peralta RJM, et al. Autophagy in inflammation, infection, neurodegeneration and cancer[J]. Int Immunopharmacol, 2014, 18 (1): 55-65. DOI:10.1016/j.intimp.2013.11.001. |
| [11] | Manley S, Williams JA, Ding WX. Role of p62/SQSTM1 in liver physiology and pathogenesis[J]. Exp Biol Med (Maywood), 2013, 238 (5): 525-538. DOI:10.1177/1535370213489446. |
| [12] | Ni HM, Bockus A, Boggess N, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity[J]. Hepatology, 2012, 55 (1): 222-232. DOI:10.1002/hep.24690. |
| [13] | Gao L, Lv G, Guo X, et al. Activation of autophagy protects against cholestasis-induced hepatic injury[J]. Cell Biosci, 2014, 4 : 47 DOI:10.1186/2045-3701-4-47. |
| [14] | Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice[J]. Gastroenterology, 2010, 139 (5): 1740-1752. DOI:10.1053/j.gastro.2010.07.041. |
| [15] | Mizushima N, Yamamoto A, Matsui M, et al. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker[J]. Mol Biol Cell, 2004, 15 (3): 1101-1111. DOI:10.1091/mbc.E03-09-0704. |
| [16] | Tan L, Dai T, Liu D, et al. Contribution of dermal-derived mesenchymal cells during liver repair in two different experimental models[J]. Sci Rep, 2016, 6 : 25314 DOI:10.1038/srep25314. |
| [17] | Sun K, Xie X, Liu Y, et al. Autophagy lessens ischemic liver injury by reducing oxidative damage[J]. Cell Biosci, 2013, 3 (1): 26 DOI:10.1186/2045-3701-3-26. |
| [18] | Lin W, Yuan N, Wang Z, et al. Autophagy confers DNA damage repair pathways to protect the hematopoietic system from nuclear radiation injury[J]. Sci Rep, 2015, 5 : 12362 DOI:10.1038/srep12362. |
| [19] | Soto-Pantoja DR, Miller TW, Pendrak ML, et al. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy[J]. Autophagy, 2012, 8 (11): 1628-1642. DOI:10.4161/auto.21562. |
| [20] | Sabin RJ, Anderson RM. Cellular senescence- its role in cancer and the response to ionizing radiation[J]. Genome Integr, 2011, 2 (1): 7 DOI:10.1186/2041-9414-2-7. |
| [21] | Pradeep K, Ko KC, Choi MH, et al. Protective effect of hesperidin, a citrus flavanoglycone, against γ-radiation-induced tissue damage in Sprague-Dawley rats[J]. J Med Food, 2012, 15 (5): 419-427. DOI:10.1089/jmf.2011.1737. |
| [22] | Chu J, Zhang X, Jin L, et al. Protective effects of caffeic acid phenethyl ester against acute radiation-induced hepatic injury in rats[J]. Environ Toxicol Pharmacol, 2015, 39 (2): 683-689. DOI:10.1016/j.etap.2015.01.020. |
| [23] | Liu X, Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases[J]. J Transl Med, 2017, 15 (1): 207 DOI:10.1186/s12967-017-1306-5. |
| [24] | Chen YX, Zeng ZC, Sun J, et al. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells[J]. J Radiat Res, 2015, 56 (4): 700-708. DOI:10.1093/jrr/rrv026. |
| [25] | Panganiban RA, Snow AL, Day RM. Mechanisms of radiation toxicity in transformed and non-transformed cells[J]. Int J Mol Sci, 2013, 14 (8): 15931-15958. DOI:10.3390/ijms140815931. |
| [26] | Ciélar-Pobuda A, Yue J, Lee HC, et al. ROS and oxidative stress in stem cells[J]. Oxid Med Cell Longev, 2017, 2017 : 5047168 DOI:10.1155/2017/5047168. |
| [27] | Friguet B, Bulteau AL, Petropoulos I. Mitochondrial protein quality control: implications in ageing[J]. Biotechnol J, 2008, 3 (6): 757-764. DOI:10.1002/biot.200800041. |
 2018, Vol. 38
2018, Vol. 38


