2. 475000 开封, 河南大学淮河医院放疗科;
3. 450000 郑州大学第一附属医院放疗科;
4. 450045 郑州, 河南省人民医院放疗科
2. Department of Radiotherapy, Huaihe Hospital of Henan University, Kaifeng 475000, China;
3. Department of Radiotherapy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China;
4. Department of Radiotherapy, Henan Provincial People's Hospital, Zhengzhou 450000, China
结直肠癌是常见的恶性肿瘤,手术切除是临床治疗结肠直癌的主要方式[1-2]。目前临床上为提高结直肠癌患者的5年生存率和局部控制率,经常辅以放、化疗[3-4]。放射治疗过程中,放射敏感性降低是影响治疗效果的主要原因,因此,探究如何提高肿瘤细胞的放射敏感性具有重要意义。有研究表明,miRNA能增加或降低肿瘤细胞的放射敏感性[5-7]。miR-885-3p在多种恶性肿瘤中异常表达,与恶性肿瘤的形成密切相关,如非小细胞肺癌[8]、结肠癌[9]等。但关于miR-885-3p对肿瘤细胞放射敏感性的相关研究较少。本研究探讨miR-885-3p在放射诱导的结直肠癌HT-29细胞中的表达量及其对细胞放射敏感性的影响,并进一步阐明其发挥作用的分子机制,为提高结直肠癌的放射敏感性提供潜在靶点。
材料与方法1.实验主要材料:HT-29细胞购自中国科学院上海细胞库,DMEM培养基、胎牛血清、青-链霉素购自美国Hyclone公司,Lipofectamine 2000购自美国赛默飞世尔有限公司,miR-885-3p模拟物、anti-miR-885-3p、PGCSIL-siAKT1-GFP vector购自广州锐博生物有限公司,聚凝胺购自北京索莱宝科技有限公司,miRNA提取试剂盒购自上海Qiagen公司,反转录试剂盒、荧光定量PCR检测试剂盒购自美国应用生物系统公司,RIPA裂解液、凋亡试剂盒购自上海碧云天生物有限公司,丝苏氨酸蛋白激酶1(serine-threonine kinase1, AKT1)3′-UTR野生型双荧光素酶报告载体购自美国Sigma公司,活化的含半胱氨酸的天冬氨酸蛋白水解酶3(cleaved cysteinyl aspartate specific proteinase 3, Cleaved Caspase-3)抗体、B细胞淋巴瘤/白血病-2(B cell lymphoma/lewkmia-2, Bcl-2)抗体、AKT1抗体购自美国Cellular Signaling Technology公司,辣根过氧化物酶标记二抗购自武汉博士德生物有限公司。
2.细胞的培养:HT-29细胞培养于含10%胎牛血清DMEM培养基,并加入1 000 U/ml的青-链霉素,放入37℃、5%CO2培养箱中,待细胞浓度大于80%,加入胰酶消化、传代。
3.细胞克隆实验:取转染后细胞,分别用0、2、4、6、8 Gy剂量的X射线照射细胞,当细胞培养板中出现明显的细胞克隆,弃去培养液,甲醇固定30 min,结晶紫染色30 min,干燥,光学显微镜下观察大于50的细胞克隆数,计算克隆形成率。利用GraphPad Prism5软件进行单击多靶模型拟合存活曲线,计算放射生物学参数平均致死量(D0)、准闭剂量(Dq)、外推值(N),计算放射增敏比(SER)。克隆形成率(PE, %)=克隆数/接种细胞数×100%,存活分数(SF)=受照射细胞PE/对照细胞PE。
4.荧光定量PCR(qPCR)检测miR-885-3p表达量:根据miRNA提取试剂盒说明书,提取细胞中总miRNA,使用反转录试剂盒将所提取的miRNA合成cDNA。以cDNA为模板,U6为参照,采用荧光定量PCR检测试剂盒进行qPCR反应。反应条件为95℃ 2 min,95℃ 15 s,60℃ 30 s,72℃ 8 min,40个循环。miR-885-3p上游引物为5′ CGCGGTATGGCACTGGTAGA 3′,下游引物为5′ AGTGCAGGGTC CGAGGTATTC 3′。实验重复3次,采用罗氏LC480软件计算Ct值,以2-ΔΔCt法计算miR-885-3p的相对表达量。
5.细胞的转染:根据Lipofectamine 2000试剂说明书将表达miR-NC、miR-885-3p、anti-miR-NC、anti-miR-885-3p、Scrambled、shAKT1和AKT1及其对照的慢病毒载体转入293T细胞进行慢病毒包装。HT-29细胞常规培养于DMEM培养基中,稳定传代后进行转染。转染前24 h,将细胞调整为1×105/ml,加入6孔板中,待细胞密度大于30%开始转染。在慢病毒感染过程中,取适量上述包装的慢病毒与500 μl DMEM培养基混合均匀,加入5 μg/ml聚凝胺,室温孵育30 min,置于6孔板中感染细胞并继续培养,12 h后更换为完全培养基,于37℃、5%CO2培养箱培养,用于后续实验。根据感染慢病毒不同,分别记为miR-NC组、miR-885-3p组、anti-miR-NC组、anti-miR-885-3p组、Scrambled组、shAKT1组。取miR-NC组、miR-885-3p组、anti-miR-NC组、anti-miR-885-3p组、Scrambled组、shAKT1组细胞,分别接受4 Gy剂量射线照射后,记为miR-NC+照射组、miR-885-3p+照射组、anti-miR-NC+照射组、anti-miR-885-3p+照射组、Scrambled+照射组、shAKT1+照射组。另取miR-885-3p组细胞分别感染过表达对照和AKT1的慢病毒,记为miR-885-3p+对照组和miR-885-3p+AKT1组。
6.细胞凋亡实验:收集转染后细胞,根据照射条件:源靶距(SSD)为100 cm,照射野10 cm×10 cm,照射剂量4 Gy,剂量率5 Gy/min,射野覆盖全细胞培养板。根据凋亡试剂盒所示,检测细胞的凋亡率。
7.蛋白质印迹法(Western blot):收集处理后24 h的细胞,吸取10 μl放射免疫沉淀法(radio-immunoprecipitation assay, RIPA)裂解液,37℃孵育10 min,提取蛋白并进行浓度测定。将适量上样缓冲液与蛋白样品混合均匀,沸水变性10 min;吸取50 μg总蛋白进行10%的十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE),然后转移至聚偏氟乙烯(PVDF)膜中,用5%的脱脂奶粉溶液封闭1 h;加入相应一抗,4℃孵育过夜,加入二抗,室温孵育2 h,曝光、拍照,分析蛋白质相对表达量。
8.双荧光素酶报告基因:通过TargetScan(http://www.targetscan.org/vert_71/)生物信息学预测软件预测miR-885-3p与AKT1的3′-端非编码区域(3′-UTR)碱基是否存在互补现象。分别构建AKT1 3′-UTR野生型双荧光素酶报告载体,并分别共转染miR-885-3p模拟物或anti-miR-885-3p,37℃、5%CO2培养箱中培养48 h,收集细胞,测定双荧光素酶的活性。
9.统计学处理:采用SPSS 22.0软件进行统计处理。所有计量数据经正态性检验均符合正态分布,结果以x±s表示。两组间数据的比较采用独立样本t检验,多组数据间的比较经方差齐性检验采用单因素方差分析,组间多重比较使用SNK-q检验。P < 0.05为差异具有统计学意义。
结果1.放射诱导HT-29细胞中miR-885-3p表达增加:qPCR检测经不同剂量(0、2、4、6、8 Gy)照射后结直肠癌HT-29细胞中miR-885-3p表达增加,其表达量分别为1.00±0.06、1.87±0.19、2.95±0.30、3.25±0.33和3.54±0.35,2、4、6、8 Gy与0 Gy比较,差异均具有统计学意义(F=46.64,P < 0.05);与2 Gy比较,4、6、8 Gy明显诱导细胞中miR-885-3p表达增加,差异具有统计学意义(F=17.85,P < 0.05);与4 Gy比较,6和8 Gy照射后细胞中miR-885-3p表达差异无统计学意义(P>0.05),故选择4 Gy进行后续实验研究。
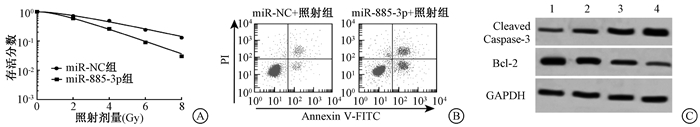
2. miR-885-3p增强HT-29细胞放射敏感性:结果见图 1、表 1。qPCR检测结果显示,转染miR-885-3p模拟物后HT-29细胞中miR-885-3p的表达量明显升高,差异具有统计学意义(t=16.11,P < 0.05);上调miR-885-3p可抑制HT-29细胞的存活分数,促进细胞凋亡,与miR-NC比较差异有统计学意义(t=12.33, P < 0.05),见图 1A、B,表 1,转染miR-885-3p增加了Cleaved Caspase-3蛋白水平,降低了Bcl-2蛋白表达量,与转染miR-NC组比较, 差异具有统计学意义(F=56.27、54.44,P < 0.05),见图 1C、表 2。
|
图 1 miR-885-3p对照射后HT-29细胞存活和凋亡的影响A.单击多靶模型拟合细胞存活曲线;B.流式细胞仪检测细胞的凋亡率;C.Western blot检测细胞中Cleaved Caspase-3和Bcl-2蛋白水平 注:1.miR-NC组;2.miR-885-3p组;3.miR-NC+照射组;4.miR-885-3p+照射组 Figure 1 The effect of miR-885-3p on survival and apoptosis of HT-29 cells after radiation A. Cell survival curve fitted by the single-hit multi-target model; B. Cell apoptosis rate detected by flow cytometry; C. Protein expressions of Cleaved Caspase-3 and Bcl-2 in the cells detected by Western blot |
|
|
表 1 转染miR-885-3p对HT-29细胞单击多靶模型参数、miR-885-3p表达及凋亡率的影响(x±s) Table 1 Effect of transfecting miR-885-3p on the parameters of single-hit multi-target model, the expression of miR-885-3p and apoptosis in HT-29 cells(x±s) |
|
|
表 2 转染miR-885-3p后HT-29细胞中凋亡相关蛋白的表达(x±s) Table 2 The expressions of apoptosis-related proteins in HT-29 cells after transfecting miR-885-3p(x±s) |
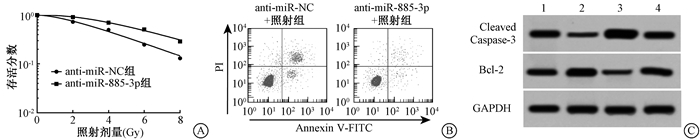
3. anti-miR-885-3p促进HT-29细胞放射抵抗:qPCR检测结果显示,转染anti-miR-885-3p模拟物后HT-29细胞中miR-885-3p的表达量明显降低,差异具有统计学意义(t=11.43,P < 0.05),见表 3;anti-miR-885-3p可促进HT-29细胞的存活,抑制细胞凋亡,与anti-miR-NC比较,差异有统计学意义(t=11.94,P < 0.05),见图 2A、B,表 3,转染anti-miR-885-3p降低了Cleaved Caspase-3蛋白水平,增加了Bcl-2蛋白表达量,与转染anti-miR-NC组比较,差异具有统计学意义(F=68.45、61.580,P < 0.05),见图 2C、表 4。
|
图 2 anti-miR-885-3p对照射后HT-29细胞存活和凋亡的影响A.单击多靶模型拟合细胞存活曲线;B.流式细胞仪检测细胞的凋亡率;C.Western blot检测细胞中Cleaved Caspase-3和Bcl-2蛋白水平 注:1. anti-miR-NC组;2. anti-miR-885-3p组;3. anti-miR-NC+照射组;4. anti-miR-885-3p+照射组;GAPDH作为内参 Figure 2 The effect of anti-miR-885-3p on survival and apoptosis of HT-29 cells after radiation A. Cell survival curve fitted by the single-hit multi-target model; B. Cell apoptosis rate detected by flow cytometry; C. Protein expressions of Cleaved Caspase-3 and Bcl-2 in the cells detected by Western blot |
|
|
表 3 转染anti-miR-885-3p对HT-29细胞单击多靶模型参数、miR-885-3p表达及凋亡的影响(x±s) Table 3 Effect of transfecting anti-miR-885-3p on the parameters of single-hit multi-target model, the expression of miR-885-3p and apoptosis in HT-29 cells(x±s) |
|
|
表 4 转染anti-miR-885-3p后各组HT-29细胞中相关蛋白的表达(x±s) Table 4 The expressions of apoptosis-related proteins in HT-29 cells after transfecting anti-miR-885-3p(x±s) |
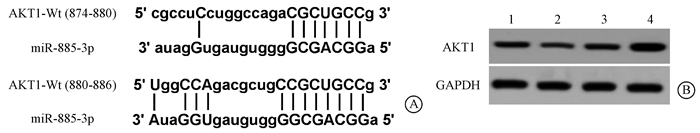
4. AKT1是miR-885-3p的靶基因:TargetScan数据库预测显示miR-885-3p与AKT1 3′UTR部分碱基互补配对,推测miR-885-3p可能对AKT1有一定的调控作用(图 3A)。双荧光素酶报告基因检测结果显示,当共转染miR-885-3p模拟物或anti-miR-885-3p进入293T细胞后,野生型AKT1 3′UTR 3′UTR报告基因的荧光素酶相对活性明显受到抑制或促进,差异具有统计学意义(F=76.05,P < 0.05),见表 5;Western blot结果显示,转染miR-885-3p模拟物或anti-miR-885-3p对AKT1蛋白水平具有明显的调控作用,差异具有统计学意义(F=44.87,P < 0.05),见图 3B、表 5。
|
图 3 miR-885-3p对HT-29细胞中AKT1的靶向调控作用A. TargetScan数据库预测miR-885-3p对AKT1的调控关系;B. Western blot检测上调或下调miR-885-3p对AKT1蛋白表达的影响 注:1. miR-NC组;2. miR-885-3p组;3. anti-miR-NC组;4. anti-miR-885-3p组 Figure 3 The targeting regulation effect of miR-885-3p on AKT1 in HT-29 cells A. the regulation between miR-885-3p and AKT1 was predicted by TargetScan database; B. the effect of up- or down-regulating miR-885-3p on AKT1 protein expression was measured by Western blot |
|
|
表 5 miR-885-3p或anti-miR-885-3p对野生型报告质粒荧光素酶相对活性及细胞中AKT1蛋白表达的影响(x±s) Table 5 Effects of miR-885-3p or anti-miR-885-3p on the relative luciferase activity of wild-type reporter plasmids and the expression of AKT1 protein in cells(x±s) |
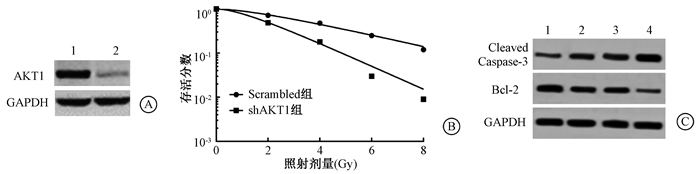
5.敲减AKT1增强HT-29的放射敏感性:Western blot检测结果显示慢病毒转染后细胞中AKT1的表达量明显降低(t=13.630,P < 0.05)(图 4A、表 6);敲减AKT1的表达量抑制HT-29细胞的存活分数,促进细胞的凋亡(t=12.95,P < 0.05),升高Cleaved Caspase-3蛋白水平(F=50.39,P < 0.05),降低Bcl-2蛋白表达量(F=45.08,P < 0.05),差异具有统计学意义(图 4B、图 4C、表 6、表 7)。
|
图 4 敲减AKT1对受照射HT-29细胞存活的影响A. Western blot检测慢病毒转染后细胞中AKT1的表达量;B.单击多靶模型拟合细胞存活曲线;C. Western blot检测细胞中Cleaved Caspase-3和Bcl-2蛋白水平 注:1.Scrambled组;2.shAKT1组;3.Scrambled+照射组;4.shAKT1+照射组 Figure 4 Effect of knockdown of AKT1 on the survival of HT-29 cells after radiation A. The expression of AKT1 in lentivirus transfected cells was detected by Western blot; B. Cell survival curve fitted by the single-hit multi-target model; C. The protein levels of Cleaved Caspase-3 and Bcl-2 in cells were measured by Western blot |
|
|
表 6 敲减AKT1对HT-29细胞单击多靶模型参数、AKT1表达及细胞凋亡的影响(x±s) Table 6 Effect of knockdown of AKT1 on the parameters of single-hit multi-target model, the expression of AKT1 and cell apoptosis in HT-29 cells(x±s) |
|
|
表 7 敲减AKT1后各组HT-29细胞中相关蛋白的表达(x±s) Table 7 The expressions of apoptosis-related proteins in HT-29 cells after knocking down AKT1(x±s) |
6.过表达AKT1逆转了miR-885-3p增强HT-29放射敏感性的作用:与miR-885-3p+Control相比,AKT1和miR-885-3p模拟物共转染入HT-29细胞显著增加细胞的存活分数,降低细胞的凋亡率,促进AKT1、Bcl-2蛋白表达,降低Cleaved Caspase 3蛋白水平,与转染前比较,差异均具有统计学意义(t=7.669、12.180、7.746、9.317,P < 0.05),见图 5、表 8。

|
图 5 过表达AKT1逆转miR-885-3p对HT-29细胞存活、凋亡及凋亡蛋白表达的影响A.单击多靶模型拟合细胞存活曲线;B.Western blot检测细胞中AKT1、Cleaved Caspase-3、Bcl-2蛋白水平 注:1. miR-NC组;2. miR-885-3p组;3. miR-885-3p+对照组;4. miR-885-3p+AKT1组 Figure 5 Overexpression of AKT1 reversed the effects of miR-885-3p on cell survival, apoptosis and the expressions of apoptotic proteins in HT-29 cells A. Cell survival curve fitted by the single-hit multi-target model; B. The protein levels of AKT1, Cleaved Caspase-3 and Bcl-2 in cells were detected by Western blot |
|
|
表 8 过表达AKT1逆转miR-885-3p对HT-29细胞中单击多靶模型参数、凋亡及凋亡蛋白表达的影响(x±s) Table 8 Overexpression of AKT1 reverses the effects of miR-885-3p on the multipile target model parameters, apoptosis and expression of apoptotic protein in HT-29 cells(x±s) |
讨论
结直肠癌是最常见的恶性肿瘤,其发病率和死亡率位居全球恶性肿瘤的前列。目前对于结直肠癌的发病机制尚不完全清楚,结直肠癌的诊断和治疗仍是临床研究面临的挑战之一。尽管手术和放疗的治疗方法得到了巨大的发展,明显提高了结直肠癌患者的长期生存率和预后,但肿瘤细胞对放疗抵抗已成为临床进一步提高放疗效果的巨大挑战。放疗抵抗包括细胞中DNA的损伤、修复以及某些基因或者信号通路的异常激活,是一个复杂的生物学过程[10-11]。放疗抵抗是导致结直肠癌患者死亡的主要原因[12]。因此,深入研究结直肠癌放射敏感性的机制,寻找与其密切相关的基因,才有望逆转放疗抵抗并进一步提高患者预后。
miRNA是一类非编码单链小分子RNA,在细胞的生长、凋亡等生物学过程中发挥重要作用[13-15]。越来越多的研究证实,miRNA不仅在肿瘤细胞的侵袭、迁移等恶性生物学过程起重要作用,而且与肿瘤细胞的放射敏感性密切相关。目前已发现,miR-124[16]、miR-133a[17]、miR-451a[18]等多种miRNA能够调控结直肠癌细胞的增殖、凋亡等生物学过程,从而调节细胞的放射敏感性。然而miR-885-3p对结肠直癌细胞放射敏感性的相关影响暂不明确。在本研究中发现,在不同剂量(0、2、4、6、8 Gy)放射诱导的HT-29细胞中,miR-885-3p的表达量逐渐增加,差异具有统计学意义,其中4 Gy剂量增加幅度较高。过表达miR-885-3p可以抑制HT-29细胞增殖、诱导其凋亡,从而增强结直肠癌细胞的放射敏感性。
近年来的研究表明,磷酸化的AKT能够激活下游多种作用底物参与肿瘤细胞的增殖、凋亡、侵袭、迁移过程,提高细胞的缺氧耐受性,增加机体血管的生成,促进细胞的放射抗性和化疗药物耐受性[19-22]。AKT在多种肿瘤组织中异常表达和磷酸化。AKT1是AKT的重要亚型之一,有研究发现,AKT1在结直肠癌中的表达量显著增加,在胃癌中也发现AKT1过度表达和活化,并在胃癌细胞的放射敏感性中发挥重要作用[23-24]。本研究通过生物信息学预测以及双荧光素酶报告基因实验表明AKT1是miR-885-3p功能性靶基因;上调和下调miR-885-3p可显著调节结直肠癌细胞中AKT1蛋白的表达量,进一步证实AKT1是miR-885-3p的直接靶基因;敲减AKT1抑制HT-29细胞的增殖、促进其凋亡,从而增强HT-29的放射敏感性;过表达AKT1逆转了miR-885-3p抑制放射诱导的细胞增殖、促进细胞凋亡作用,表明AKT1是miR-885-3p调节结直肠癌细胞放射敏感性的功能性靶基因。
综上所述,本实验结果发现,miR-885-3p通过调控AKT1增强结直肠癌HT-29细胞的放射敏感性,为临床提高结直肠癌放射敏感性提供了一个重要的实验依据和靶点。
利益冲突 全体作者无利益冲突,排名无争议,未因进行该研究而接受任何不正当的职务和财务利益作者贡献声明 李全营、吴大鹏设计研究方案、收集数据后统计并起草论文;顾浩、贺志宽、汪洋、葛政协助完成实验;秦长江、王伟指导、监督实验进行,修改论文
| [1] |
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(3): 177-193. DOI:10.3322/caac.21395 |
| [2] |
Cohen R, Svrcek M, Duval A, et al. Immune checkpoint inhibitors for patients with colorectal cancer:mismatch repair deficiency and perspectives[J]. Colorectal Cancer, 2017, 6(1): 23-31. DOI:10.2217/crc-2017-0004 |
| [3] |
刘娟. 同步化疗与序贯化疗对直肠癌术后患者局部控制率和生存率的作用[J]. 医药前沿, 2018, 8(20): 227-228. Liu J. Effects of synchronous chemotherapy and sequential chemotherapy on local control rate and survival rate of postoperative rectal cancer patients[J]. J Front Med, 2018, 8(20): 227-228. DOI:10.3969/j.issn.2095-1752.2018.20.195 |
| [4] |
王宏伟, 邢宝才. 结直肠癌肝转移术前药物治疗进展[J]. 中国实用外科杂志, 2016, 36(4): 447-450. Wang HW, Xing BC. Advances in preoperative drug therapy for hepatic metastasis of colorectal cancer[J]. Chin J Practical Surg, 2016, 36(4): 447-450. DOI:10.7504/cjps.issn.1005-2208.2016.04.21 |
| [5] |
Chen X, Xu Y, Liao X, et al. Plasma miRNAs in predicting radiosensitivity in non-small cell lung cancer[J]. Tumour Biol, 2016, 37(9): 11927-11936. DOI:10.1007/s13277-016-5052-8 |
| [6] |
Hao C, Xu X, Ma J, et al. MicroRNA-124 regulates the radiosensitivity of non-small cell lung cancer cells by targeting TXNRD1[J]. Oncol Lett, 2017, 13(4): 2071-2078. DOI:10.3892/ol.2017.5701 |
| [7] |
Hou W, Song L, Zhao Y, et al. Inhibition of Beclin-1-mediated autophagy by microrna-17-5p enhanced the radiosensitivity of glioma cells[J]. Oncol Res, 2017, 25(1): 43-53. DOI:10.3727/096504016X14719078133285 |
| [8] |
Shin S, Cha HJ, Lee EM, et al. Alteration of miRNA profiles by ionizing radiation in A549 human non-small cell lung cancer cells[J]. Int J Oncol, 2009, 35(1): 81-86. DOI:10.3892/ijo_00000315 |
| [9] |
Xiao F, Qiu H, Cui H, et al. MicroRNA-885-3p inhibits the growth of HT-29 colon cancer cell xenografts by disrupting angiogenesis via targeting BMPR1A and blocking BMP/Smad/Id1 signaling[J]. Oncogene, 2015, 34(15): 1968-1978. DOI:10.1038/onc.2014.134 |
| [10] |
Mueller AK, Lindner K, Hummel R, et al. MicroRNAs and their impact on radiotherapy for cancer[J]. Radiat Res, 2016, 185(6): 668-677. DOI:10.1667/RR14370.1 |
| [11] |
Pajic M, Froio D, Daly S, et al. miR-139-5p modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense[J]. Cancer Res, 2018, 78(2): 501-515. DOI:10.1158/0008-5472.CAN-16-3105 |
| [12] |
Yang P, Yang Y, An W, et al. The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway[J]. J Gastroenterol Hepatol, 2017, 32(4): 837-845. DOI:10.1111/jgh.13606 |
| [13] |
Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development[J]. Biochim Biophys Acta, 2016, 1859(1): 169-176. DOI:10.1016/j.bbagrm.2015.06.015 |
| [14] |
Ma ZL, Hou PP, Li YL, et al. MicroRNA-34a inhibits the proliferation and promotes the apoptosis of non-small cell lung cancer H1299 cell line by targeting TGFβR2[J]. Tumour Biol, 2015, 36(4): 2481-2490. DOI:10.1007/s13277-014-2861-5 |
| [15] |
Reddy KB. MicroRNA (miRNA) in cancer[J]. Cancer Cell Int, 2015, 15: 38. DOI:10.1186/s12935-015-0185-1 |
| [16] |
Lin SM, Xia Q, Zhang YQ, et al. miR-124 regulates radiosensitivity of colorectal cancer cells by targeting PRRX1[J]. J South Med Univ, 2016, 36(8): 1110-1116. DOI:10.3969/j.issn.1673-4254.2016.08.15 |
| [17] |
Yang QS, Jiang LP, He CY, et al. Up-Regulation of MicroRNA-133a Inhibits the MEK/ERK signaling pathway to promote cell apoptosis and enhance radio-sensitivity by targeting EGFR in esophageal cancer in vivo and in vitro[J]. J Cell Biochem, 2017, 118(9): 2625-2634. DOI:10.1002/jcb.25829 |
| [18] |
Ruhl R, Rana S, Kelley K, et al. microRNA-451a regulates colorectal cancer proliferation in response to radiation[J]. BMC Cancer, 2018, 18(1): 517. DOI:10.1186/s12885-018-4370-1 |
| [19] |
Bao L, Liu F, Guo HB, et al. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway[J]. Tumour Biol, 2016, 37(8): 11365-11374. DOI:10.1007/s13277-016-5013-2 |
| [20] |
Umesalma S, Nagendraprabhu P, Sudhandiran G. Ellagic acid inhibits proliferation and induced apoptosis via the Akt signaling pathway in HCT-15 colon adenocarcinoma cells[J]. Mol Cell Biochem, 2015, 399(1-2): 303-313. DOI:10.1007/s11010-014-2257-2 |
| [21] |
Leszczynska KB, Foskolou IP, Abraham AG, et al. Hypoxia-induced p53 modulates both apoptosis and radiosensitivity via AKT[J]. J Clin Invest, 2015, 125(6): 2385-2398. DOI:10.1172/JCI80402 |
| [22] |
Tolcher AW, Patnaik A, Papadopoulos KP, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma[J]. Cancer Chemother Pharmacol, 2015, 75(1): 183-189. DOI:10.1007/s00280-014-2615-5 |
| [23] |
许欣, 姚冬颖. 结直肠癌中Girdin和Akt1蛋白的表达及临床意义[J]. 河北医药, 2016, 42(15): 2259-2261, 2266. Xu X, Yao DY. Expression and clinical significance of Girdin and Akt1 in colorectal carcinoma[J]. Hebei Med, 2016, 42(15): 2259-2261, 2266. DOI:10.3969/j.issn.1002-7386.2016.15.005 |
| [24] |
Sasaki T, Yamashita Y, Kuniyasu H. AKT plays a crucial role in gastric cancer[J]. Oncol Lett, 2015, 10(2): 607-611. DOI:10.3892/ol.2015.3260 |
 2018, Vol. 38
2018, Vol. 38


