2. 350014 福州, 福建医科大学附属肿瘤医院 福建省肿瘤医院 病理科;
3. 362000 泉州, 福建医科大学附属第二医院病理科
2. Fujian Cancer Hospital, Fujian Medical University, Fuzhou 350014, China;
3. Department of Pathology, Second Affiliated Hospital of Fujian Medical University Cancer Hospital, Quanzhou 362000, China
结直肠癌是世界范围内的常见恶性肿瘤之一,其中直肠癌占30%~35%[1]。早期直肠癌患者可无明显症状,就诊时多为局部晚期。通过术前新辅助放化疗,可以使10%~30%的直肠癌患者实现病理完全缓解,获得更好的预后[2-3]。Dhadda等[4]报道,肿瘤部分消退的患者同样也具有更好的无病生存率(disease-free survival, DFS)和总生存率(overall survival, OS)。然而,接受新辅助放化疗的局部晚期直肠癌患者中,仅45%的患者可使肿瘤降期[5],大部分患者肿瘤完全没反应或者有轻微反应。如何运用有效的肿瘤消退分级(TRG)标准来评价疗效和预测患者的预后,具有重要意义。Mandard[6]最早在食管癌中提出将TRG分为5级,可判断新辅助治疗的疗效。本研究采用Mandard评价标准将TRG分为肿瘤消退明显组(TRG1+2) 和肿瘤消退不明显组(TRG3+4+5),然后利用磁共振扩散加权成像(DWI)技术测量放疗后肿瘤表观弥散系数(apparent diffusion coefficient,ADC),探讨TRG与肿瘤ADC值之间的关系。
资料与方法1.一般资料:选取2004年6月1日至2015年9月15日福建省肿瘤医院收治的直肠癌患者106例为研究对象,男72例,女34例,均经病理证实为直肠癌T3~T4或者伴有淋巴结转移且接受术前放疗,并将术后病理切片根据Mandard评分标准重新进行肿瘤消退分级。选取病例基本情况详见表 1。
|
|
表 1 106例局部晚期直肠癌患者的基本情况 Table 1 General clinicopathological characteristics of 106 patients with locally advanced rectal cancer |
2.手术:106例患者均接受根治性切除术,按照全直肠系膜切除术的标准实施。手术切缘距离肿瘤至少达2 cm以上,外科医生依据肿瘤的大小、部位等具体情况决定手术的术式。手术的方式包括保留肛门的经腹低位前切除术(LAR)或不保留肛门的腹会阴联合切除术(APR),行APR的患者,均行回肠造口术。51例患者行LAR手术,55例行APR手术。
3.放疗:所有患者均接受术前放疗,放疗方案分长程放疗和短程放疗。放疗体位取仰卧位,使用网罩固定患者体位。CT将扫描的盆腔图像传至Oncentra工作平台上,在CT重建图像上勾画出临床靶区及危及器官。长程放疗组处方剂量:50 Gy/25次,2 Gy/次,5 d/周;短程放疗组处方剂量:25 Gy/5次,5 Gy/次,5 d/周。
4.化疗:58例患者放疗期间同步接受以氟尿嘧啶类药物为基础的化疗,其中30例患者放疗同期接受卡培他滨单药口服化疗;28例患者放疗同期接受奥沙利铂+氟尿嘧啶+亚叶酸钙(FOLFOX)方案化疗。64例患者术后接受以氟尿嘧啶类药物为基础的辅助化疗,其中32例患者放疗同期接受FOLFOX方案化疗;32例患者术后接受奥沙利铂+卡培他滨方案化疗。
5. Mandard肿瘤消退评分标准[6]:Mandard TRG将肿瘤消退分为5级,TRG1:没有肿瘤细胞,仅见纤维组织,病理完全缓解;TRG2:肿瘤消退明显,仅见散在单个的肿瘤细胞或小巢团状的肿瘤;TRG3:肿瘤组织中伴明显的纤维坏死的炎症区域,纤维成分占50%以上;TRG4:肿瘤轻微消退,残留的肿瘤细胞超过纤维坏死的炎症区域;TRG5:肿瘤无消退,完全没有变化。将Mandard TRG 1+2分为消退明显组,Mandard TRG 3+4+5分为消退不明显组。
6.随访:对所有患者进行术后随访,2年内每3个月随访1次,2年以上每6个月随访1次,随访主要采用电话随访。以总生存时间为研究终点,定义为手术日至患者死亡或随访截至的日期。
7.统计学处理:采用SPSS 19.0软件进行分析。计数资料采用χ2检验,生存分析采用Kaplan-Meier法,应用Log-Rank检验和Cox模型分析预后因素。P<0.05为差异有统计学意义。
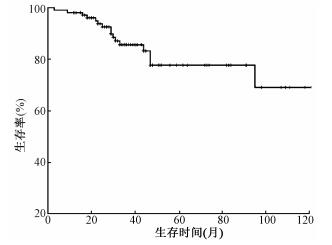
结果1.患者的总生存率及预后影响因素:全组1、2、3年OS分别为98.1%(95%CI 95.6%~100.0%),85.6%(95%CI 80.0%~93.2%)和77.7%(95%CI 66.7%~88.7%)。中位OS时间为34个月(3~145个月),见图 1。单因素分析提示,年龄、化疗、pT分期、pN分期、分化程度、脉管癌栓以及TRG可能对OS有影响(χ2=5.057、4.842、3.945、4.112、5.429、5.114、8.110,P<0.05,表 2)。将上述因素纳入Cox多因素分析,结果显示,仅分化程度和TRG是OS的独立预后因素(χ2=5.221、6.563,P<0.05,表 3)。
|
图 1 106例局部晚期直肠癌患者的总生存情况 Figure 1 Overall survival of 106 patients with locally advanced rectal cancer |
|
|
表 2 106例局部晚期直肠癌患者的总生存单因素分析 Table 2 Univariate analysis of risk factors affecting the overall survival in 106 patients with locally advanced rectal cancer |
|
|
表 3 106例局部晚期直肠癌患者的总生存多因素分析 Table 3 Multivariate analysis of risk factors affecting the overall survival in 106 patients with locally advanced rectal cancer |
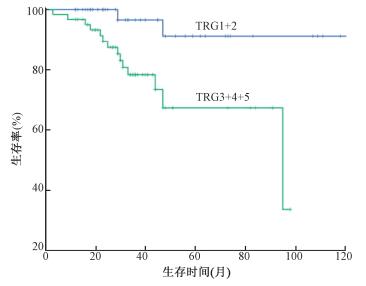
术前长程放疗和短程放疗的5年OS分别为83.1%(95%CI 76.1%~100.0%)和68.4%(95% CI 50.8%~86.0%),差异无统计学意义(P>0.05)。肿瘤中高分化和低未分化的5年OS分别为89.5%(95%CI 81.5%~97.5%)和58.1%(95% CI 50.8%~86.0%),差异有统计学意义(χ2=5.221,P<0.05)。TRG1+2和TRG3+4+5的5年OS分别为91.2%(95%CI 79.2%~100.0%)和67.4%(95%CI 50.0%~84.8%),差异有统计学意义(χ2=6.563,P<0.05,图 2)。
|
图 2 TRG对106例局部晚期直肠癌患者总生存的影响 Figure 2 Overall survival of 106 patients with locally advanced rectal cancer according to TRG |
2.TRG1+2和TRG3+4+5两组患者之间的相关因素比较:结果列于表 4。由表 4可知,术前放疗方式、术前化疗、病理类型、分化程度、大体类型、脉管癌栓和放疗后肿瘤ADC值对TRG有影响(χ2=18.139、16.206、5.130、4.189、10.149、4.340、10.630,P<0.05)。
|
|
表 4 TRG1+2和TRG3+4+5两组患者之间的相关因素比较 Table 4 Comparison of clinicopathological characteristics of patients between TRG1+2 and TRG3+4+5 |
4.放疗后肿瘤ADC值预测TRG1+2的ROC曲线:放疗后肿瘤ADC值作为诊断是否TRG1+2的指标,绘制ROC曲线列于图 3。结果显示,放疗后肿瘤ADC值阈值取最靠近ROC曲线左上点1.7×10-3 mm2/s,诊断TRG1+2的灵敏度为0.938,特异度为0.441,ROC曲线下面积为0.707(95%CI 0.555~0.859);放疗后肿瘤ADC值预测TRG1+2疗效:阳性预测值为87.5%,阴性预测值为61.8%。

|
图 3 放疗后肿瘤ADC值预测TRG1+2的ROC曲线 Figure 3 The ROC curve of the tumor ADC after radiotherapy to predict TRG1+2 |
讨论
由于直肠癌早期症状不明显,大部分患者就诊时已是局部晚期。而局部晚期直肠癌若仅接受单纯根治性手术治疗,复发率可达20%~60%,5年生存率仅50%[7]。研究显示,新辅助治疗(neoadjuvant therapy,NAT)使直肠癌治疗的结果得到了很大的提高,不仅提高低位直肠癌的保肛率,而且显著改善局部控制率[8]。目前,NAT联合全直肠系膜切除术已经成为局部晚期直肠癌的标准治疗方式。然而由于个体差异,NAT的治疗效果并不一样。文献报道,NAT可以使cT3-4直肠癌患者的病理完全缓解率达9%~29%[9]。大部分直肠癌患者术前放疗后,病理只是部分消退。而肿瘤的消退程度与预后存在密切关系,因此准确的疗效评价标准显得尤为重要。它可以用来预测患者新辅助治疗的效果,为后续的治疗提供依据,实现精准的个体化治疗。本研究通过长期随访观察106例局部晚期直肠癌,初步研究结果显示,年龄、新辅助放化疗、pT分期、pN分期、分化程度、脉管癌栓和TRG是OS预后影响因素;分化程度和TRG是OS独立预后因素;长程放化疗组和短程放疗组之间的OS差异无统计学意义;放疗后肿瘤ADC值以1.7×10-3 mm2/s作为阈值,可较好预测术前放疗后的肿瘤消退情况。
术前放疗有两种方式,北欧国家多采用术前短程放疗[9],而美国等西方国家多采用术前长程放疗[10]。长程放化疗和短程放疗之间的随机对照研究显示,两组之间的疗效差异无统计学意义[11]。本研究结果显示,短程放疗组和长程放化疗组的5年总生存率分别为77.0%和83.1%,差异无统计学意义。Bujko等[12]研究报道,短程放疗组和长程放化疗组的总生存率差异无统计学意义,长程放疗和短程放疗后的pCR率分别为16%和0.7%。长程放化疗取得较高的pCR率可能与同步化疗、较高的放疗剂量及更长的间隔时间有关[13]。另有国内学者进行了改良术前短程放疗(30 Gy/10次)的尝试,结果显示,5年OS为65.3%(95%CI,63.1%~67.5%),pCR率为2.6%[14]。本研究结果表明,接受长程放疗和短程放疗后的pCR率分别为19.4%(12/62) 和2.3%(1/44),差异有统计学意义,与上述研究结果相似。
直肠癌术前放疗后,肿瘤病理改变不一,有些完全病理缓解,有些肿瘤完全没消退或者消退不佳。而病理完全消退或部分消退的患者比病理消退不佳的患者具有更好的无病生存[15]。相比于肿瘤降期和肿瘤体积缩小,从组织学层面来判断肿瘤放疗后的消退更为准确[16]。1994年,Mandard[6]首次提出TRG的概念。直肠癌新辅助放疗后约20%的患者可以达到病理完全缓解,且这部分患者的预后更好[2]。本研究根据Mandard评估标准对肿瘤消退进行分级,结果发现,TRG1+2和TRG3+4+5两组患者之间的OS差异有统计学意义,说明Mandard TRG可作为患者放疗后预后的预测指标。本研究对两组患者的临床病理因素对比发现,术前放疗方式、病理类型、分化程度、大体类型以及脉管癌栓和TRG相关,提示造成不同患者对NAT的反应敏感性不同的原因可能与这些因素有关。
DWI是通过水分子的弥散运动作为成像基础,比形态学更早地发现病理生理学改变,可以反应组织微观结构的改变。作为一种MR功能成像方法,DWI已广泛应用于直肠癌的治疗当中[17]。研究表明,直肠癌及转移淋巴结的ADC值较低[18]。本研究通过测量不同患者肿瘤放疗后的ADC值发现,肿瘤消退明显组(TRG1+2) 患者的ADC值明显高于肿瘤消退不明显组(TRG3+4+5),该结果与国外研究结果相似[19]。原因可能是肿瘤细胞密度高,增强了细胞膜对水分子运动的受到限制,当肿瘤细胞受到放化疗破坏时,细胞膜的完整性受到破坏,水分子的扩散从而增加,从而使ADC值上升。本研究的放疗后肿瘤ADC值只有50例,样本量明显偏少,且易受感兴趣区的大小和位置的影响。但初步结果显示,放疗后肿瘤ADC值以1.7×10-3 mm2/s作为阈值,能够较好地预测直肠癌术前放疗后的TRG1+2。
利益冲突 无作者贡献声明 邵凌东负责论文的撰写;李金銮负责数据的整理和分析;杜开新、贺俊彦参与数据的分析;陈少华和廖雪洪负责病理的肿瘤消退诊断;彭清琴参与数据的收集;吴君心负责研究设计和最终版本的修订
| [1] | Jemal A, Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61 (2): 69-90. DOI:10.3322/caac.20107. |
| [2] | Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer[J]. 2012, 99(7):918-928. DOI:10.1002/bjs.8702. |
| [3] | Zorcolo L, Rosman AS, Restivo A, et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival:a meta-analysis[J]. Ann Surg Oncol, 2012, 19 (9): 2822-2832. DOI:10.1245/s10434-011-2209-y. |
| [4] | Dhadda AS, Dickinson P, Zaitoun AM, et al. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer[J]. Eur J Cancer, 2011, 47 (8): 1138-1145. DOI:10.1016/j.ejca.2010.12.006. |
| [5] | Kuremsky JG, Tepper JE, Mcleod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer[J]. Int J Radiat Oncol Biol Phy, 2009, 74 (3): 673-688. DOI:10.1016/j.ijrobp.2009.03.003. |
| [6] | Mandard JC. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma.Clinicopathologic correlations[J]. Cancer, 1994, 73 (1): 2680-2686. |
| [7] | Cammà C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer:A meta-analysis[J]. JAMA, 2000, 284 (8): 1008-1015. DOI:10.1001/jama.284.8.1008. |
| [8] | Dhadda AS, Dickinson P, Zaitoun AM, et al. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer[J]. Eur J Cancer, 2011, 47 (8): 1138-1145. DOI:10.1016/j.ejca.2010.12.006. |
| [9] | van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer:12-year follow-up of the multicentre, randomised controlled TME trial[J]. Lancet Oncol, 2011, 12 (6): 575-582. DOI:10.1016/S1470-2045(11)70097-3. |
| [10] | Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer:results of the German CAO/ARO/AIO-94 randomized phase Ⅲ trial after a median follow-up of 11 years[J]. 2012, 30(16):1926-1933. DOI:10.1200/JCO.2011.40.1836. |
| [11] | Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer:trans-tasman radiation oncology group trial 01.04[J]. J Clin Oncol, 2012, 30 (31): 3827-3833. DOI:10.1200/JCO.2012.42.9597. |
| [12] | Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer[J]. Brit J Surg, 2006, 93 (10): 1215-1223. DOI:10.1002/bjs.5506. |
| [13] | Bujko K, Nasierowskaguttmejer A, Wyrwicz L, et al. Neoadjuvant treatment for unresectable rectal cancer:an interim analysis of a multicentre randomized study[J]. Radiother Oncol J Eurn Societ Therapeut Radiol Oncol, 2013, 107 (2): 171-177. DOI:10.1016/j.radonc.2013.03.001. |
| [14] | Peng YF, Yu WD, Pan HD, et al. Tumor regression grades:Potential outcome predictor of locally advanced rectal adenocarcinoma after preoperative radiotherapy[J]. World J Gastroenterol, 2015, 21 (6): 1851-1856. DOI:10.3748/wjg.v21.i6.1851. |
| [15] | Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited:updated results of the CAO/ARO/AIO-94 trial[J]. J Clin Oncol, 2014, 32 (15): 1554-1562. DOI:10.1200/JCO.2013.54.3769. |
| [16] | Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment[J]. Fron Oncol, 2013, 3 : 262 DOI:10.3389/fonc.2013.00262. |
| [17] | Lambrecht M, Vandecaveye V, De Keyzer F, et al. The value of diffusion-weighted magnetic resonance imaging in the prediction and early evaluation of response to neoadjuvant treatment in rectal cancer[J]. Radiother Oncol, 2010, 96 (1): 444 DOI:10.1016/j.ijrobp.2010.12.063. |
| [18] | Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer:added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo-and radiation therapy1[J]. Radiology, 2009, 253 (1): 116-125. DOI:10.1148/radiol.2532090027. |
| [19] | Lambrecht M, Vandecaveye V, De KF, et al. Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer:preliminary results[J]. Int J Radiat Oncol Biol Phys, 2012, 82 (82): 863-870. DOI:10.1016/j.ijrobp.2010.12.063. |
 2017, Vol. 37
2017, Vol. 37


