2. 210006 南京医科大学附属南京医院泌尿外科
2. Department of Urology, Affiliated Nanjing Hospital, Nanjing Medical University, Nanjing 210006, China
抗血管生成治疗成为近年来肿瘤治疗的研究热点,其中Avastin是一种人源化全长单克隆IgG抗体,可阻止血管内皮生长因子(vascular endothelial growth factor,VEGF)与其受体在血管内皮表面结合,抑制血管内皮增生和新生血管形成[1-2]。而VEGF靶向放射性显像剂因治疗药物Avastin的竞争而影响其摄取和显像,VEGF与整合素αvβ3的交互应答对细胞多种功能(增殖和迁移)共同发挥作用[3-4],为应用整合素αvβ3靶向显像剂评价VEGF靶向治疗(包括抗新生血管治疗)肿瘤疗效提供理论基础[5-7],前期一步法合成的99Tcm-3P4-RGD\-2整合素αvβ3靶向好,肿瘤99Tcm-3P4-RGD\-2每克组织百分注射剂量率(%ID/g)摄取与整合素αvβ3 表达水平呈正相关[8]。因此,本研究拟探讨99Tcm-3P4-RGD\-2 microSPECT/CT显像早期评价肿瘤Avastin抗新生血管治疗疗效的应用价值。
材料与方法1.实验材料:放射性高锝酸钠溶液由南京森科公司提供,Hynic-3P4-RGD2试剂盒由美国普渡大学刘爽教授馈赠,Avastin购自美国Genentech公司,羊血清、 啮齿动物抗整合素CD61抗体、 大鼠抗CD31抗体、 Cy3标记羊抗啮齿动物二抗和荧光素(FITC)标记羊抗大鼠二抗均购自美国BD Biosciences公司。人前列腺癌细胞(PC-3)及人脑恶性胶质母细胞瘤细胞系(U87MG)由本实验室保存。U-SPECT/CT(荷兰Milabs公司)显像在上海市肿瘤研究所完成。高效液相色谱(HPLC)仪购自日本岛津公司,γ放射性活度测定仪购自美国Perkin Elmer公司,荧光显微镜(BX-51)购自日本Olympus公司。
2. 实验动物:BALB/c裸鼠,雄性,4~5周龄,(16±2)g。购自上海斯莱克实验动物公司,合格证号:SXXK(沪)2012-0002。
3. 99Tcm-Hynic-3P4-RGD2的合成:一步法制备99Tcm-3P4-RGD2,具体制备方法见文献[8-9],用生理盐水配制放射性浓度为370 MBq/ml备用。
4. 细胞培养及荷瘤裸鼠模型建立:人脑胶质瘤细胞(U87MG,高整合素αvβ3表达)培养基为含10%胎牛血清(FBS)的最低基础培养液,人前列腺癌细胞(PC-3,低整合素αvβ3表达)培养基为含10% FBS的RPMI 1640,分别置于37℃、 5%CO2培养箱培养,收集并调整细胞浓度为5×107/ml,取裸鼠皮下注射0.1 ml(5×107/ml)人脑胶质瘤细胞(U87MG)或人前列腺癌细胞(PC-3),SPF环境下饲养至肿瘤直径达6.0~7.0 mm。
5. Avastin腹腔注射治疗:按照随机数字表法,每种肿瘤分为治疗组和生理盐水对照组。PC-3治疗组和U87MG治疗组,每组4只:给予Avastin腹腔注射,每日0.05 mg/g,隔日1次,共计8次/只,同时测定裸鼠体重,动态监测治疗药物的不良反应。PC-3对照组和U87MG对照组,每组4只:腹腔注射同体积生理盐水。4组每组取2只荷瘤鼠,在药物治疗前1 d,治疗后3、 5、 10和15 d,分别尾静脉注射3.7 MBq 99Tcm-3P4-RGD2后1 h进行microSPECT/CT显像,以上实验重复3次。
6. microSPECT/CT显像及图像处理:荷脑胶质瘤(U87MG)裸鼠尾静脉注射3.7 MBq 99Tcm-3P4-RGD2,异氟烷空气麻醉箱麻醉,SPECT扫描参数为:时间30 min,75次投射,CT扫描电压45 kV,电流500 μA。microSPECT图像重建通过有序最大期望值法(OSEM)程序完成,microCT图像重建通过锥体束滤波反投影算法完成,将microSPECT数据根据体素与microCT数据完全匹配得到新的microSPECT数据,进行microSPECT/CT图像融合,调整获得较好的对比度和低噪音图像。
7. microSPECT/CT显像定量分析:根据文献[8, 10]方法,在microSPECT/CT融合图像上,逐层勾画肿瘤边界获得肿瘤感兴趣区,通过PMOD软件 (瑞士PMOD公司)计算肿瘤体积及肿瘤组织放射性摄取计数,并进行时间放射性衰减校正,根据microSPECT放射性计数与放射性活度的转换关系[11],计算肿瘤放射性摄取(%ID和%ID/g),每个显像时间点,每组分别取荷瘤鼠1只,处死,解剖分离肿瘤,进行免疫组织化学检查。
8. 肿瘤组织免疫荧光检查:将肿瘤组织切成5 μm厚标本,干燥和冰丙酮固定,羊血清封闭30 min,加一抗啮齿动物抗整合素β3抗体(1∶100)和大鼠抗CD31抗体(1∶100)后,室温避光1~4 h,磷酸盐缓冲液(PBS)冲洗后加Cy3标记羊抗-Hamster二抗和荧光素(FITC)标记羊抗大鼠二抗,室温避光1~4 h,PBS冲洗,甘油封片,荧光显微镜下观察图像并通过Image J软件定量组织CD31和整合素β3表达水平,所有获取的图像放大倍数均为100倍,每个肿瘤组织切片随机选择30个视野,肿瘤整合素β3表达水平即荧光密度表达为荧光面积(红色)占视野整个面积的百分比。
9. 统计学处理:肿瘤体积及放射性摄取呈正态分布,以x±s表示,采用GraphPad Prism 5.0软件进行分析, 肿瘤体积及放射性摄取 组间比较采用t检验。P<0.05为差异有统计学意义。
结果1.Avastin对脑胶质瘤及前列腺癌的抑瘤效果:结果见表 1。U87MG治疗组肿瘤体积在第5天略增加,与治疗前1 d相比,差异无统计学意义(P>0.05)。治疗后10 d,U87MG治疗组肿瘤体积为明显小于U87MG对照组(t=5.81,P<0.05),且随时间延长差异增大。PC-3治疗组和PC-3对照组肿瘤体积随时间延长均缓慢增加,相同时间点两组肿瘤体积差异无统计学意义。治疗15 d时,PC-3治疗组(21.3±2.64)g和PC-3对照组(20.9±3.04)g荷瘤鼠体重差异无统计学意义(t=23.9,P>0.05),提示药物的不良反应不明显,而U87MG对照组(18.5±2.41)g低于U87MG治疗组的(22.4±3.22)g。
|
|
表 1 荷人前列腺癌及人脑胶质瘤裸鼠经Avastin或生理盐水干预后肿瘤体积变化(cm3,x±s) Table 1 Tumor volume changes after administration of Avastin or saline in nude mice bearing prostate cancer and glioma xenograft(cm3,x±s) |
2.microSPECT/CT显像定量肿瘤99Tcm-3P4-RGD2放射性摄取:行99Tcm-3P4-RGD2microSPECT/CT显像见图 1。计算肿瘤体积及放射性摄取,结果列于表 2。治疗前1 d,U87MG治疗组肿瘤放射性摄取(%ID/g)高于PC-3治疗组;Avastin治疗3 d,U87MG治疗组脑胶质瘤(%ID/g)明显小于U87MG对照组(t=3.26, P<0.05),且随着治疗进行,U87MG治疗组脑胶质瘤放射性摄取(%ID和%ID/g)均逐渐减小;U87MG对照组脑胶质瘤放射性摄取(%ID)从5 d开始逐渐增加,但%ID/g随着肿瘤体积增加而降低。PC-3治疗组和PC-3对照组在治疗前后99Tcm-3P4-RGD2 microSPECT/CT显像肿瘤部位放射性摄取(%ID/g)均较低,差异无统计学意义(P>0.05),且在治疗后15 d,PC-3治疗组microCT肿瘤体积小于PC-3对照组,但差异无统计学意义(P>0.05)。
|
|
表 2 microSPECT-CT显像定量脑胶质瘤对99Tcm-3P4-RGD2放射性摄取情况(x±s) Table 2 Tumor uptake of 99Tcm-3P4-RGD2quantified by microSPECT/CT in glioma bearing nude mice(x±s) |
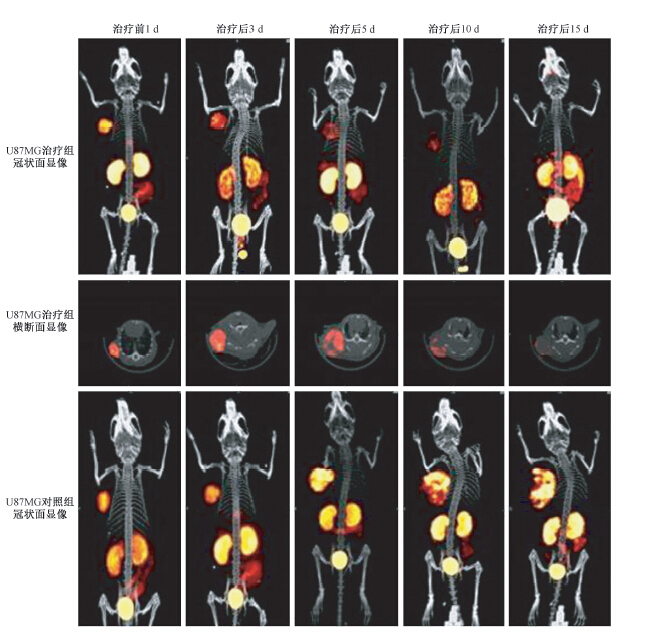
|
图 1 荷人脑胶质瘤裸鼠在抗新生血管药物治疗前及治疗后不同时间99Tcm-3P4-RGD2 microSPECT/CT显像 Figure 1 99Tcm-3P4-RGD2microSPECT/CT images performed at different time before and after Avastin administration in glioma bearing nude mice |
3. 前列腺癌及脑胶质瘤Avastin治疗过程中肿瘤整合素β3表达:结果示于图 2。PC-3前列腺癌组织新生血管内皮细胞及肿瘤细胞整合素表达水平低,Avastin治疗3 d,新生血管内皮细胞及肿瘤细胞整合素β3表达水平变化不明显。而脑胶质瘤治疗前肿瘤细胞及新生血管内皮细胞均高表达,与对照组相比,Avastin治疗3 d整合素β3表达水平降低,其中以新生血管内皮细胞整合素受体表达降低尤为显著,肿瘤细胞整合素表达呈稳定性降低,而治疗第5天时,同第3天治疗组和生理盐水对照组相比,脑胶质瘤可见新生血管内皮细胞整合素受体β3表达量显著减少。绘制散点图示于图 3,两者呈较好的线性相关y=0.499 1x-0.243 8(R2=0.811 7)。
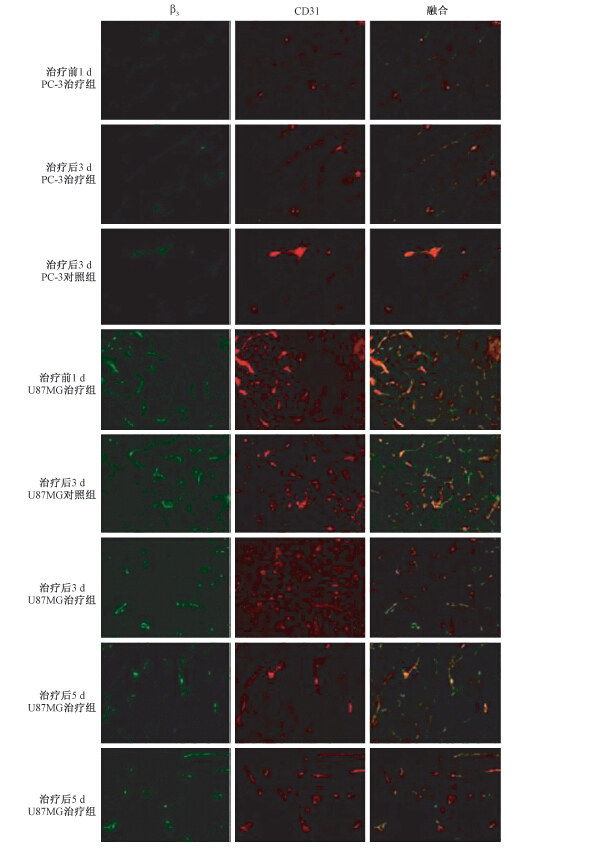
|
图 2 前列腺癌(PC-3)和脑胶质瘤(U87MG)治疗前1 d及治疗后3、 5 d肿瘤整合素β\-3和CD31免疫荧光检查 ×100 注:红色表示整合素β3的分布和表达(包括肿瘤细胞和新生血管内皮细胞),绿色表示血管CD31分布和表达 (包括成熟血管和新生血管),叠加图片黄色表示新生血管上整合素β3 的表达 Figure 2 Immunofluorescence examination of integrin β\-3 and CD31 expression in PC-3 prostate cancer and U87MG glioma before and after Avastin administration ×100 |
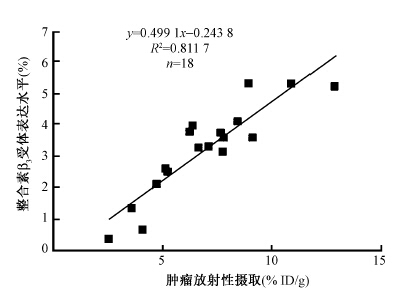
|
图 3 脑胶质瘤对99Tcm-3P4-RGD2的摄取 与肿瘤整合素β3表达水平的关系 Figure 3 Linear relationship between glioma uptake of 99Tcm-3P4-RGD2and integrin β\-3 expression |
讨论
肿瘤治疗效果评价既往多采用世界卫生组织(WHO)或实体肿瘤的疗效评价标准(RECIST)[12],即依赖体积变化评估疗效,抗血管生成药物主要作用于肿瘤新生血管,但肿瘤体积变化常远迟于血供抑制,也得到本实验结果的证实,因此,既往标准不适用于抗血管药物[13]。影像学手段如CT灌注成像和对比剂增强MRI主要利用抗血管生成药物抑制新血管生成使肿瘤退缩及瘤组织代谢抑制[14-15],在CT、 MRI上表现为灌注参数和代谢状况的变化,但其对肿瘤治疗效果的评价价值有待验证。整合素α\-vβ\-3高表达于肿瘤新生血管内皮细胞表面,而在成熟血管内皮细胞表面几乎不表达,有效评估肿瘤组织整合素α\-vβ\-3受体表达水平,为抗血管生成治疗患者的筛选和疗效监测提供了重要手段[17-19]。近年来放射性核素及荧光标记RGD多肽已用于肿瘤显像[18-19],而99Tcm-3P4-RGD2作为整合素受体靶向显像剂,一步合成,价廉易得,具有广泛的应用前景。microSPECT/CT显像通过肿瘤感兴趣区的勾画定量肿瘤体积和放射性摄取,为有效评价肿瘤部位整合素受体表达提供数据支持,实验鼠脏器小,对射线吸收少,可忽略不计,人体组织对射线的吸收明显,影响放射性核素的精确定量,但目前设备可通过CT衰减校正得到有效解决。本实验抗新生血管治疗过程中,脑胶质瘤对99Tcm-3P4-RGD2的特异性摄取与肿瘤整合素α\-vβ\-3受体表达水平呈线性相关。因此,SPECT/CT显像为临床有效定量评价肿瘤99Tcm-3P4-RGD2摄取及评价整合素α\-vβ\-3受体表达变化提供依据。实验结果显示,Avastin可有效抑制脑胶质瘤生长,尤其是新生血管生成情况更为显著,实验中U87MG治疗组新生血管内皮细胞整合素α\-vβ\-3受体表达降低为其提供数据支持,且在治疗早期(第3天),整合素β\-3受体降低主要表现为肿瘤新生血管内皮细胞,而肿瘤细胞整合素受体β\-3表达降低缓慢,在治疗后第5天可见肿瘤细胞和新生血管内皮细胞表面整合素受体表达水平均显著降低,与99Tcm-3P4-RGD2显像结果吻合,考虑与新生血管生成减少,肿瘤细胞处于相对缺血缺氧状态,而抑制了肿瘤生长相关,也在一定程度上证实Avastin肿瘤抗血管治疗疗效和99Tcm-3P4-RGD2放射性摄取减少的机制,为其用于Avastin抗肿瘤疗效的早期评价提供数据支持。本研究结果以新生血管密度较低和整合素受体表达水平较低的前列腺癌作为对照,抗新生血管治疗显示肿瘤体积变化与生理盐水组无明显差异,免疫荧光结果证实其整合素受体表达水平持续较低,与生理盐水组差异不明显,提示前列腺癌(PC-3)不是Avastin 抗新生血管治疗的适应证,而99Tcm-3P4-RGD2显像对监测前列腺癌的疗效价值有限[20]。
18F-FDG PET显像评价肿瘤治疗效果多有报道,但肿瘤治疗早期(7 d内)却表现出"闪烁显像",可能机制为在肿瘤细胞增生受到抑制的同时常伴炎症细胞如巨噬细胞浸润,从而表现为肿瘤部位18F-FDG摄取增加[21]。有文献报道,放射性核素标记RGD在肿瘤部位的摄取几乎不受肿瘤治疗过程中所伴炎性细胞浸润的影响[22]。Haubner等[23]对化疗的肺癌患者同期行18F-FDG和18F-Glacto-RGD PET显像,结果显示在治疗开始2周,肿瘤葡萄糖代谢无显著变化,而肿瘤对18F-Glacto-RGD的摄取减少了20%。本实验中,在整合素受体高丰度表达的脑胶质瘤抗新生血管治疗过程中,99Tcm-3P4-RGD2显像发现肿瘤放射性摄取变化明显早于肿瘤体积变化,U87MG治疗组在抗新生血管治疗后第3天,肿瘤对99Tcm-3P4-RGD2的摄取(%ID和%ID/g)即出现明显降低,在治疗后15 d几乎无明显摄取,而肿瘤体积几乎无明显降低,因此在抗新生血管治疗早期疗效评价上显示出明显优势,99Tcm-3P4-RGD2 microSPECT/CT显像动态监测肿瘤整合素受体表达水平和变化,为其用于早期评价肿瘤抗新生血管治疗疗效提供数据支持。而在低整合素α\-vβ\-3受体表达的前列腺癌,99Tcm-3P4-RGD2 microSPECT/CT显像的应用价值较低。
利益冲突 本研究还由南京市医学科技发展基金(QRX11253)资助,本人与本人家属、 其他研究者,未因进行该研究而接受任何不正当的职务或财务利益,在此对研究的独立性和科学性予以保证作者贡献声明 邵国强负责药物标记、 动物实验和文章书写;杨瑞和梁凯负责显像和细胞培养;姚晓晨和崔璨负责治疗、 动物一般情况观察及免疫组织化学检测;王峰、 王自正负责课题设计、 统计分析和文章修改
| [1] | Yamatodani T, Holmqvist B, Kjellén E, et al. Using intravital microscopy to observe bevacizumab-mediated anti-angiogenesis in human head and neck squamous cell carcinoma xenografts[J]. Acta Otolaryngol , 2012, 132 (12) : 1324-1333 DOI:10.3109/00016489.2012.699195 |
| [2] | Fischer CV, Mans V, Horn M, et al. The antiproliferative effect of bevacizumab on human tenon fibroblasts is not mediated by vascular endothelial growth factor inhibition[J]. Invest Ophthalmol Vis Sci , 2016, 57 (11) : 4970-4977 DOI:10.1167/iovs.16-19938 |
| [3] | Mahabeleshwar GH, Feng W, Reddy K, et al. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis[J]. Circ Res , 2007, 101 (6) : 570-580 DOI:10.1161/CIRCRESAHA.107.155655 |
| [4] | Soldi R, Mitola S, Strasly M, et al. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2[J]. EMBO J , 1999, 18 (4) : 882-892 DOI:10.1093/emboj/18.4.882 |
| [5] | Somanath PR, Ciocea A, Byzova TV. Integrin and growth factor receptor alliance in angiogenesis[J]. Cell Biochem Biophys , 2009, 53 (2) : 53-64 DOI:10.1007/s12013-008-9040-5 |
| [6] | Battle MR, Goggi JL, Allen L, et al. Monitoring tumor response to antiangiogenic sunitinib therapy with 18F-fluciclatide, an 18F-labeled αVbeta3-integrin and αV beta5-integrin imaging agent[J]. J Nucl Med , 2011, 52 (3) : 424-430 DOI:10.2967/jnumed.110.077479 |
| [7] | Haubner R, Wester HJ. Radiolabeled tracers for imaging of tumor angiogenesis and evaluation of anti-angiogenic therapies[J]. Curr Pharm Des , 2004, 10 (13) : 1439-1455 DOI:10.2174/1381612043384745 |
| [8] | Fu T, Qu W, Qiu F, et al. 99mTc-3P-RGD2 micro-single-photon emission computed tomography/computed tomography provides a rational basis for integrin αvβ3-targeted therapy[J]. Cancer Biother Radiopharm , 2014, 29 (9) : 351-358 DOI:10.1089/cbr.2014.1622 |
| [9] | 孙昱, 王任婕, 高识, 等. 99Tcm-3P4-RGD2 SPECT显像对孤立性肺结节的诊断价值[J]. 中华核医学与分子影像杂志 , 2013, 33 (1) : 24-28 Sun Y, Wang RI, Gao S, et al. Diagnostic value of solitary pulmonary nodules using 99Tcm-3P4-RGD2 scintigraphy[J]. Chin J Nucl Med Mol Imaging , 2013, 33 (1) : 24-28 DOI:10.3760/cma.j.issn.2095-2848.2013.01.006 |
| [10] | Vaneycken I, Govaert J, Vincke C, et al. In vitro analysis and in vivo tumor targeting of a humanized, grafted nanobody in mice using pinhole SPECT/micro-CT[J]. J Nucl Med , 2010, 51 (7) : 1099-1106 DOI:10.2967/jnumed.109.069823 |
| [11] | 刘伟, 孟庆乐, 杨瑞, 等. microSPECT/CT显像定量评价肿瘤99mTc-3P4-RGD2摄取及整合素αβ表达水平的实验研究[J]. 南京医科大学学报(自然科学版) , 2014, 8 : 1124-1130 Liu W, Meng QL, Yang R, et al. Quantitive analysis of 99Tcm-3P4-RGD2 uptake and tumor integrin αVβ3 expression in glioma bearing nude mice with microSPECT/CT imaging[J]. Acta Univ Med Nanjing , 2014, 8 : 1124-1130 |
| [12] | 陈智伟, 廖美琳, 陈玉蓉, 等. WHO标准和RECIST标准评价肺癌化疗疗效的比较[J]. 循证医学 , 2004, 4 (2) : 83-84 Chen ZW, Liao ML, Chen YR, et al. Measuring response of chemotherapy in lung cancer:WHO versus RECIST criteria[J]. J Evid-Based Med , 2004, 4 (2) : 83-84 DOI:10.3969/j.issn.1671-5144.2004.02.011 |
| [13] | Prasad SR, Saini S, Sumner JE, et al. Radiological measurement of breast cancer metastases to lung and liver:comparison between WHO (bidimensional) and RECIST (unidimensional) guidelines[J]. J Comput Assist Tomogr , 2003, 27 (3) : 380-384 DOI:10.1097/00004728-200305000-00014 |
| [14] | Fournier LS, Oudard S, Thiam R, et al. Metastatic renal carcinoma:evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT[J]. Radiology , 2010, 256 (2) : 511-518 DOI:10.1148/radiol.10091362 |
| [15] | Hahn OM, Yang C, Medved M, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma[J]. J Clin Oncol , 2008, 26 (28) : 4572-4578 DOI:10.1200/JCO.2007.15.5655 |
| [16] | Miller JC, Pien HH, Sahani D, et al. Imaging angiogenesis:applications and potential for drug development[J]. J Natl Cancer Inst , 2005, 97 (3) : 172-187 DOI:10.1093/jnci/dji023 |
| [17] | Cai W, Rao J, Gambhir SS, et al. How molecular imaging is speeding up antiangiogenic drug development[J]. Mol Cancer Ther , 2006, 5 (11) : 2624-2633 DOI:10.1158/1535-7163.MCT-06-0395 |
| [18] | Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis[J]. J Nucl Med , 2008, 49 (Suppl 2) : 113S-128S DOI:10.2967/jnumed.107.045922 |
| [19] | Shi J, Zhou Y, Chakraborty S, et al. Evaluation of in-labeled cyclic rgd peptides:effects of peptide and linker multiplicity on their tumor uptake, excretion kinetics and metabolic stability[J]. Theranostics , 2011, 1 : 322-340 DOI:10.7150/thno/v01p0322 |
| [20] | Cheng Z, Wei R, Wu C, et al. Ex-vivo biodistribution and micro-PET/CT imaging of 18F-FDG, 18F-FLT, 18F-FMISO, and 18F-AlF-NOTA-PRGD2 in a prostate tumor-bearing nude mouse model[J]. Nucl Med Commun , 2015, 36 (9) : 914-921 DOI:10.1097/MNM.0000000000000339 |
| [21] | Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo:high accumulation in macrophages and granulation tissues studied by microautoradiography[J]. J Nucl Med , 1992, 33 (11) : 1972-1980 |
| [22] | Sun X, Yan Y, Liu S, et al. 18F-FPPRGD2 and 18F-FDG PET of response to Abraxane therapy[J]. J Nucl Med , 2011, 52 (1) : 140-146 DOI:10.2967/jnumed.110.080606 |
| [23] | Haubner R, Beer AJ, Wang H, et al. Positron emission tomography tracers for imaging angiogenesis[J]. Eur J Nucl Med Mol Imaging , 2010, 37 (Suppl 1) : S86-103 DOI:10.1007/s00259-010-1503-4 |
 2017, Vol. 37
2017, Vol. 37


