Polonium-210(210Po) occurs in natural environment[JP] at trace levels as part of the 238U decay chain. As a pure α emitter,210Po is one of the most radiotoxic nuclides. With wide dispersion in the environment,210Po enters human body via food chains,thus being a serious threat to human health. 210Po was known as one of the most important sources of the internal dose received by humans from foods[1]. For example,it was reported that 210Po contributed 75% of dose due to the α-radiation in marine organisms and contributed 50% of the internal α-ray irradiation dose to people[2]. The maximum permissible human body-burden of ingested 210Po was 1.1×103 Bq[3]. Concerning the public health,it is important to ensure that the contaminant levels in diet will not exceed the permissible limits[4]. To address this heightened concern and effectively respond to 210Po radiological emergency,the capability to rapidly analyze large numbers of samples in a variety of matrices is necessary for risk assessment and post incident decision making. 210Po was detected in a large variety of samples,including soil,water,marine organisms,tobacco leaves,cigarettes,urine,and biological materials[5,6],especially in seafood such as mollusc[7,8]. The commonly used methods included α spectrometer measurement after source preparation through spontaneous deposition[9]. However,the traditional methods of sample pretreatment process is complex and time consuming. So it is necessary to establish a simple routine environmental analysis method.
In this study,a new method was developed to determine 210Po in seafood using large area grid ionization chamber α spectrometry.
Microwave digestion system (Preekem Scientific Instruments Co.[KG-*4],China),ultrasonic cleaner (Tianjin Science Instrument Co.[KG-*4],Ltd,China),209Po (1.178 Bq/ml,Beijing Research Institute of Uranium Geology),standard source of 237Np,239Pu and 241Am (National Institute of Metrology,China),ultrapure water purification system (Milli-Q,USA),sodium dodecyl sulfate (CP,Tianjin Fuchen Chemical Reagents Factory),gelatin (CP,Xilong Chemical Co.[KG-*4],Ltd) and formaldehyde solution (AR,Xilong Chemical Co.[KG-*4],Ltd),HNO3 (MOS,Tianjin Fengchuan Chemical Reagent Co.[KG-*4],Ltd) and H2O2 (CP,Tianjin Fengchuan Chemical Reagent Co.[KG-*4],Ltd). 2. Sample preparation
Clam samples were collected from market in Beijing. The soft tissues were taken out,dried at 105℃,and grinded into powder. 0.1-0.5 g of powder was weighted into Teflon vessel,HNO3 (5 ml) and H2O2 (2 ml) were added into each vessel,and 209Po standard solution was added as the tracer. Samples were digested for 3 min at 105℃,using a microwave system with maximum power of 1 500 W,and then heated to 200℃ for 3 min. After the vessel being cooled to ambient temperature,the acid solution was transferred into weighted beaker,evaporated to dryness at 60-80℃[1],and then beaker was weighed again. Then 50 ml of ultrapure water was added,disperse sample sdution under ultrasonic for 40-60 min,then 1‰ formaldehyde (0.5 ml) and 2×10-3 gelatin (0.5 ml) were added into sample solution[10]. Then the sample solution was transferred directly into the dish ( 25 cm),the surface of which was treated with 5% sodium dodecyl sulfate (SDS),and the sample dish were then put in the vacuum oven on a horizontal tray,evaporated to dryness at 50-60℃. The counting source prepared in this way can be put in large-area grid ionization chamber directly.
Following the above sample preparation procedure,0.1,0.2,0.3,0.4 and 0.5 g of clam powder was weighted,respectively,for the purpose of obtaining effective thickness of sample source.
3.Spectrometry
The sample sources were counted using large-area grid ionization chamber (Oradela Model 8210A). The chamber filled with commercially available mixed gas of 90% Ar and 10% CH4 to about 48.26 kPa[11]. Standard source (237Np- 239Pu- 241Am mixed source, 25 cm) was used for energy calibration and efficiency determination (237Np 4 789 keV,239Pu 5 156 keV,241Am 5 485 keV,and activity 136.4 Bq). The minimum detectable activity (MDA) was calculated by the following formula[12]:
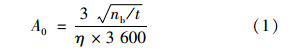
A comparative method for the measurement of 210Po in clam samples was employed using the multi-channel α spectrometer (OCTE-Plus,USA),sample source was prepared through cupper disc spontaneous deposition[13].
1.Detection efficiency,MDA and recovery
The φ 25 cm blank dish was counted for 24 h,while mixed standard source for 30 min,to obtain detection efficiency 33% of the large-area grid ionization chamber α spectrometry. MDA was 9.870×10-4 Bq. 209Po standard solution was spiked in clam powder and the present method was used to prepare source. After 24 h measurement,the recovery was 98.5%- 98.6%.
2. Alpha energy spectrum
All five sample sources could present complete shape of peaks at the same energy range in 4 880-5 330 keV. The α energy spectrums were compared,and the results showed that the peak shapes were both larger and more tailing while the sample source thickness increased. And thickness of sample source (258.14 g/cm2,0.3 g dry) had the sharpest peak than others.
3.The effect of sample source thickness
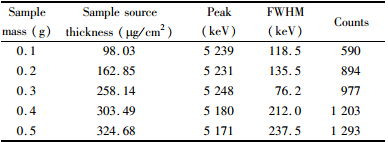
The counting results obtained from five different thicknesses of samples were compared in Table 1. The source thickness was equal to the sample residue mass divided by counting dish’s area. The count increased with the source thickness as a result of sample mass. At the same specific activity,the larger mass means higher activity. When the source thickness was 258.14 μg/cm2,the full width at half maximum (FWHM) was lower than others.
| Table 1 Peak,full width at half maximum (FWHM) and counts for different thickness sources |
The dry clam samples were analyzed using both multi-channel α spectrometer and large-area grid ionization chamber α spectrometry. The spontaneous deposition source (φ 2 cm) measured using the multi-channel α spectrometer. The average activity of 210Po was 0.056 4 Bq/g (dry sample). The clam powder sample 0.3 g was weighted,digested and detected for 36 h in the same chamber using the method presented in this study. The activity of 210Po was 0.057 5 Bq/g (dry sample). The results indicated that 210Po activities obtained using both methods were nearly identical.
In this study,a new method was developed to digest seafood samples using microwave system and determine 210Po with large-area grid ionization chamber α spectrometry. This method features easy preparation and small quantity of samples,without separation step. In order to prepare the counting source as evenly as possible,the SDS was used to treat sample dish in attempt to achieve hydrophilia of inner surface,so as to allow homogeneous distribution and evaporation of sample solution by aid of horizontal tray[14]. Compared with the plating method as adopted the National Standards (GB 14883.5\|1994)[13],the quantity of sample was reduced from 5-50 g to 0.2-0.5 g. The digestion process was able to avoid large amount of foam and the loss of fat in some samples.
The developed method in this study,using microwave digestion,needs less than 1 g of sample. Digestion and cooling process could be completed in less than 1 h,which accelerated the digestion of fats and helped to obtain clear solution at last. Moreover,many samples could be processed in a parallel way. The literature reviewed the large-area cylindrical grid ionization chamber had higher sensitivity than semiconductor α spectrometer,because of the lower high quality coefficient,which was in direct proportion to MDA[12] . Large screen grid ionization chamber’s lower limit of detection for a counting time 1 000 min at a confidence level of 95% was calculated to be 0.012 pCi(1 Ci=3.7×1010 Bq)[11].
At the stage of sample source preparation,the disc diameter of 25 cm was used in the present method whereas in range of 2-2.5 cm in plating method. Thus,the probe area of the present method was 100 times bigger than that of plating method. About the sample source thickness,the increasing of sample mass lead to the increase of the source thickness[14]. At the same specific activity the larger mass means higher activity. So,more counts could be obtained from the chamber. With the same activity and measurement time,its resolution decreased with source thickness. At the same thickness and measurement time,the counts increased with activity. For this reason,sufficient counts cannot be obtained from 0.1 and 0.2 g of samples,without peaks of 210Po found. The 0.4 and 0.5 g of thicker samples resulted in higher self-absorption of α particles and α spectrum peaks tailing[11]. Therefore,when using the large-area grid ionization chamber to measure α spectrum,the thickness of the source is main influencing factor.
Above all,the method developed for measurement of seafood could achieve higher recovery (above 98%) and lower MDA (9.870×10-4 Bq),using small quantity of sample (0.2-0.5 g of dry sample). The present method is useful for measurement of other biological or environmental samples.
Acknowledgments
Dr. Wang Tiejian was specially acknowledged for his donation of facility to multi-channel α spectrometer measurement at Beijing Research Institute of Uranium Geology.
| [1] | Planinšek P, Benedik L, Smodiš B. Comparison of various dissolution techniques for determination of 210Po in biological samples[J]. Appl Radiat Isot, 2013, 81(11): 53-56. |
| [2] | Holtzman RB. Natural levels of lead-210, polonium-210 and radium-226 in humans and biota of the Arctic[J]. Nature, 1966, 210(5041): 1094-1097. |
| [3] | Lee HW, Wang JJ. Annual dose of Taiwanese from the ingestion of 210Po in oysters[J]. Appl Radiat Isot, 2013, 73: 9-11. |
| [4] | Cunha IL, Bueno L, Fávaro DIT, et al. Analysis of 210Pb and 210Po in Brazilian foods and diets[J]. J Radioanal Nucl Chem, 2001, 247(2): 447-450. |
| [5] | Guérin N, Dai X. Rapid preparation of polonium counting sources for α spectrometry using copper sulfide microprecipitation[J]. Anal Chem, 2013, 85(13): 6524-6529. |
| [6] | Desideri D, Roselli C, Meli MA. Intake of 210Po, 234U and 238U radionuclides with wine in Italy[J]. Food Chem Toxicol, 2010, 48(2): 650-657. |
| [7] | Gouvea RC, Santos PL, Dutra IR. Lead-210 and polonium-210 concentrations in some species of marine molluscs[J]. Sci Total [LL]Environ, 1992, 112(2-3): 263-267. |
| [8] | Dahlgaard H. Polonium-210 in mussels and fish from the Baltic-North Sea estuary[J]. J Environ Radioact, 1996, 32(95): 91-96. |
| [9] | Lin Z, Wu Z. Analysis of polonium-210 in food products and bioassay samples by isotope-dilution α spectrometry[J]. Appl Radiat Isot, 2009, 67(5): 907-912. |
| [10] | 李树棠, 杨大亭, 刘玉莲, 等. 大面积低水平α放射性能谱源的制备和样品的测定方法[J]. 核化学与放射化学, 1989, (3): 149-155. |
| [11] | Hötzl H, Winkler R. Experiences with large-area frisch grid chambers in low-level α spectrometry[J]. Nucl Instrum Methods Phys Res, 1984, 223(84):290-294. |
| [12] | 潘自强. 电离辐射环境检测与评价[M]. 北京:原子能出版社, 2007: 312-313. |
| [13] | 中华人民共和国卫生部. GB 14883.5-1994, 中华人民共和国国家标准, 食品中放射性物质检测钋-210的测定[S]. 北京: 中国标准化出版社, 2005. |
| [14] | Biehl R, Paschke M, Pilwat G. Preparation of large-area sources with uniform layers for the spectrometry of α-emitting nuclides[J]. J Radioanal Nucl Chem, 1999, 242(3): 721-725. |
 2015, Vol. 35
2015, Vol. 35