γ-H2AX是目前公认的DNA双链断裂(DSBs)的分子标志物。人外周血淋巴细胞γ-H2AX分析作为辐射生物剂量计的可行性已得到许多研究的证实[1,2,3,4]。与双着丝粒染色体畸变估算外照射生物剂量相比,它具有灵敏度高和高通量筛选的优点,可估算的最低剂量达1 mGy[5],并可快速对核/辐射事故受照人群进行伤情分类诊断和剂量估算[6]。本研究采用免疫荧光技术和外照射方法,比较γ射线照射诱导大鼠和人外周血淋巴细胞γ-H2AX焦点形成和消除动力学,以及DSBs修复核心分子ATM和DNA-PKcs激活效应的差别[7,8],比较大鼠整体照射和离体照射外周血淋巴细胞诱导γ-H2AX焦点形成的差异,并探索其剂量-效应关系,为采用γ-H2AX这一指标研究辐射生物剂量估算方法提供动物模型,同时为阐述人外周血淋巴细胞DSBs修复机制提供实验依据。
1. 实验动物及人血样来源:SPF级雄性Sprague Dawley(SD)大鼠72只,体重(200±20)g,由复旦大学实验动物中心提供,许可证号:SCXK(沪)2009-0019。选择3名无烟酒嗜好、无毒物及射线接触史的健康成年人抽取静脉血,签署知情同意书。
[JP+1] 2.主要试剂与仪器:RPMI 1640细胞培养液、胎牛血清(FBS)和10 000 U/ml青霉素-10 mg/ml链霉素溶液均购自美国Invitrogen公司;人外周血淋巴细胞分离液购自加拿大Cedarlanelabs公司;大鼠外周血淋巴细胞分离液购自天津市灏洋生物制品科技有限责任公司;多聚赖氨酸购自美国Sigma公司;兔源γ-H2AX(S139)一抗购自美国Cell Signaling公司;鼠源pATM(S1981)、pDNA-PKcs(T2609)一抗购自美国Abcam公司;Alexa fluor 488驴抗兔IgG和Alexa fluor 555驴抗鼠IgG均购自美国Life公司;UltraCruz抗荧光淬灭剂(DAPI)购自美国Santa Cruz公司。荧光显微镜(BX51型)为日本Olympus公司产品。137Cs γ辐照装置(γ-cell-40型)为加拿大Nordion公司生产,吸收剂量率为0.74 Gy/min。
3. 淋巴细胞的分离培养与照射:大鼠经10%水合氯醛麻醉、消毒后,心脏采血2~4 ml于肝素抗凝管中。健康成年人肘正中静脉穿刺采血约10 ml于肝素抗凝管中。按照厂家说明书进行外周血淋巴细胞分离,分离后用含10%FBS和血浆的RPMI 1640培养液重悬细胞,采用137Cs源进行γ射线离体照射,吸收剂量为0.25、0.5、0.75、1和2 Gy,照射后的样品置于37℃、5%CO2,饱和湿度条件下培养至0.5、2、6和24 h。整体照射的大鼠于2 Gy γ射线照射后6 h麻醉取血,分离淋巴细胞进行免疫荧光检测。离体照射和整体照射实验均设置未照射的空白对照组。
4. 细胞免疫荧光实验检测γ-H2AX、pATM(S1981)和pDNA-PKcs(T2609)焦点的形成:分别于0.5 Gy γ射线照射后30 min,2、6和24 h或0.25~1 Gy γ射线照射后2 h收集培养的大鼠和人淋巴细胞悬液,同时每个时间点取未照射细胞作为空白对照。调整每个样品的细胞密度约为6×105/ml,用细胞离心机将细胞贴附于预先用多聚赖氨酸包被的玻璃载玻片上,经4%(W/V)多聚甲醛固定,0.5% Triton X-100/磷酸盐缓冲液(PBS)通透,10%小牛血清/PBS 37℃封闭后,加入1%牛血清白蛋白(BSA)稀释的一抗4℃孵育过夜;PBS漂洗后加入荧光二抗室温避光孵育1 h;漂洗后加含DAPI的抗淬灭剂并封片,在荧光显微镜下观察并记录淋巴细胞核中绿色γ-H2AX焦点数和红色pATM(S1981)或pDNA-PKcs(T2609)焦点数,每个样品计数100~500个细胞,换算为每个细胞含有的焦点数;同时观察γ-H2AX焦点与pATM(S1981)焦点、γ-H2AX焦点与pDNA-PKcs(T2609)焦点的共定位情况,采用以下公式计算:共定位比例=两种焦点的共定位数×2/两种焦点数之和。
5.统计学处理:数据用x±s表示。采用Origin 8.0软件进行时间-效应和剂量-效应曲线拟合。采用SPSS 20.0软件进行分析,两组间比较采用独立样本t检验。P<0.05为差异有统计学意义。
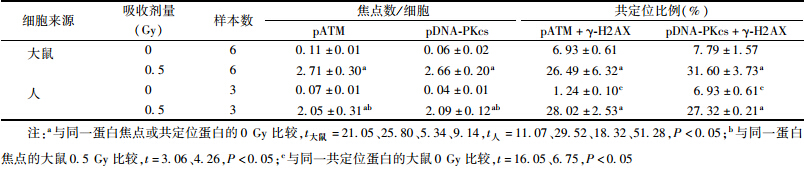
1.离体照射诱发大鼠和人淋巴细胞γ-H2AX焦点形成和消除过程:由表 1可知,正常大鼠外周血淋巴细胞γ-H2AX焦点的本底值较低,与人外周血淋巴细胞差异无统计学意义;0.5 Gy γ射线照射后30 min,大鼠和人淋巴细胞γ-H2AX焦点形成数均达最高峰(t=62.64、28.52,P<0.05);随着照射后时间的延长,胞核中γ-H2AX焦点数快速下降:照射后2 h,大鼠和人淋巴细胞γ-H2AX焦点数分别显著降低为最大值的71%和73%(t=9.09、4.22,P<0.05),照后6 h分别显著降低为最大值的22%和23% (t=45.96、14.80,P<0.05),至照射后24 h仍可检测到γ-H2AX残留焦点,但焦点数仅约为最大值的3%~8%,接近本底值。大鼠和人淋巴细胞γ-H2AX焦点形成和消除过程具有很好的一致性。
| 表 1 γ射线照射诱发大鼠和人外周血淋巴细胞γ-H2AX焦点形成的时间-效应关系(焦点/细胞,x±s) |
2. 离体照射诱发大鼠和人淋巴细胞pATM、pDNA-PKcs焦点形成及其与γ-H2AX焦点的共定位:由表 2可知,正常大鼠和人外周血淋巴细胞pATM (S1981)和pDNA-PKcs (T2609)焦点的本底值均较低,且在两种淋巴细胞之间差异无统计学意义,但是大鼠淋巴细胞pATM (S1981)或pDNA-PKcs (T2609) 焦点与γ-H2AX焦点共定位的本底值显著高于人淋巴细胞的本底值(t=16.05、6.75,P<0.05)。γ射线照射后30 min,大鼠和人淋巴细胞pATM (S1981)与pDNA-PKcs (T2609) 焦点数均较其本底值显著升高(t大鼠=21.05、25.80,P<0.05; t人=11.07、29.52,P<0.05),且这两种焦点分别与γ-H2AX焦点共定位的比例显著增加,均为26%~32%(t大鼠=5.34、9.14,P<0.05;t人=18.32、51.28,P<0.05),这3种焦点的形态在大鼠和人淋巴细胞中亦相一致(图 1);但是γ射线照射后30 min,大鼠淋巴细胞pATM(S1981)和pDNA-PKcs(T2609)焦点形成数明显高于人淋巴细胞的表达水平(t=3.06、4.26,P<0.05)。
| 表 2 离体照射诱发大鼠和人外周血淋巴细胞pATM、pDNA-PKcs焦点形成及与γ-H2AX焦点的共定位(x±s) |
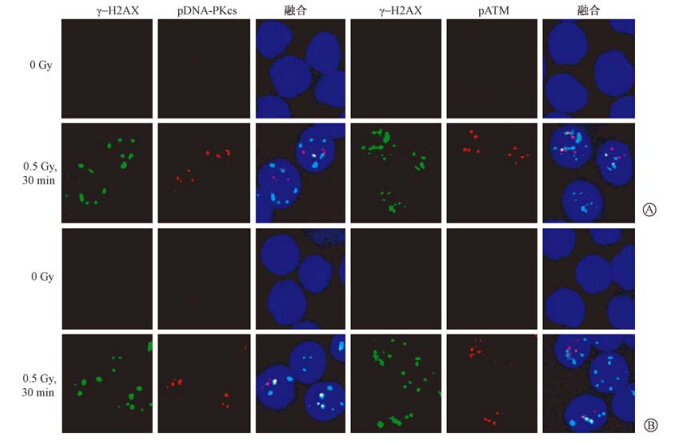 | 图 1 离体照射诱发大鼠和人外周血淋巴细胞γ-H2AX、pATM、pDNA-PKcs焦点形成的代表性图像 免疫荧光染色 ×100 A.大鼠;B.人 |
3. 整体照射和离体照射诱导大鼠淋巴细胞γ-H2AX焦点形成的比较:大鼠经2 Gy γ射线整体照射后6 h,外周血淋巴细胞γ-H2AX焦点数较本底值(0.08±0.03)焦点/细胞明显增加至(3.12±0.39)焦点/细胞(t=15.55,P<0.05);相同剂量离体照射大鼠淋巴细胞后6 h诱导γ-H2AX焦点数为(3.08±0.03)焦点/细胞,亦显著高于本底值(0.07±0.02)焦点/细胞(t=229.87,P<0.05),但与整体照射诱导的γ-H2AX焦点数差异无统计学意义。
4. 离体照射诱导大鼠外周血淋巴细胞γ-H2AX焦点形成的剂量-效应关系:γ射线照射后2 h,大鼠外周血淋巴细胞 γ-H2AX焦点形成数随吸收剂量增大而显著增加,在0~1.0 Gy范围内,γ-H2AX焦点数与剂量呈线性关系,拟合方程为Y=6.809D+0.089 8,斜率为6.81焦点/Gy,R2=0.995。
近年来,人外周血离体研究和采用恒河猴和小型猪动物模型研究已证实,γ-H2AX焦点分析可用于均匀外照射和局部照射的生物剂量估计[9,10,11,12,13,14]。尽管该方法在实际应用中因焦点消除而存在一定的局限性,但是作为估算生物剂量最敏感的方法和用于人群高通量筛选的特性,被国内外生物剂量学研究领域所肯定[15]。本研究采用外照射方法,通过与人外周血淋巴细胞相比较,对大鼠用作γ-H2AX焦点生物剂量计研究动物模型的可行性进行了验证。结果表明,γ射线照射诱导大鼠外周血淋巴细胞γ-H2AX焦点形成和消除动力学与人外周血淋巴细胞基本一致,并且焦点的形态及荧光强度在大鼠和人淋巴细胞之间亦无明显区别。通过检测在DNA损伤应答(DDR)中调控γ-H2AX形成的上游关键分子ATM[16],以及在G0/G1期细胞的DSBs修复中起主导作用的非同源末端连接(NHEJ)的核心分子DNA-PKcs的激活状态[8],进一步比较大鼠和人淋巴细胞DSBs的修复机制,结果显示,辐射能诱导大鼠和人淋巴细胞ATM S1981位点与DNA-PKcs T2609位点的磷酸化而使其激活,尽管辐射诱导人淋巴细胞pATM (S1981) 和pDNA-PKcs (T2609)焦点形成的增加明显低于大鼠外周血淋巴细胞,但辐射诱导pATM (S1981)或pDNA-PKcs (T2609)焦点分别与γ-H2AX焦点共定位比例的显著增加,在大鼠和人淋巴细胞之间无明显差异。由于修复蛋白在DSBs处共定位是有效修复的关键,因此,这可能是辐射诱导大鼠和人外周血淋巴细胞γ-H2AX焦点形成和消除动力学相一致的重要原因。人淋巴细胞pATM(S1981)或pDNA-PKcs(T2609) 与γ-H2AX焦点共定位的本底值远低于大鼠淋巴细胞,表明辐射诱导人淋巴细胞DSBs修复蛋白共定位的反应可能强于大鼠淋巴细胞。有研究报道,ATM直接参与了NHEJ介导的DSBs修复,它通过磷酸化DNA-PKcs T2609位点,并与DNA-PKcs自磷酸化协同作用,促进DSBs修复[17],这可能是本实验观察到辐射诱导pATM (S1981)焦点与pDNA-PKcs (T2609) 焦点形成数相近的原因。另有研究报道,在DDR过程中,除了激活的ATM使H2AX磷酸化之外[16],DNA-PKcs还能通过不依赖ATM的方式直接磷酸化H2AX,也能通过其他信号通路间接调节H2AX磷酸化水平[18],而且只有≤25%DSBs依靠ATM信号来进行修复[19],这些都可能是本实验观察到的辐射诱导pATM (S1981)焦点与pDNA-PKcs (T2609)焦点形成数显著低于γ-H2AX焦点数的原因。
离体和整体效应相一致,且与吸收剂量之间具有较好的剂量-效应关系,是生物剂量计应具备的基本条件。本实验进一步证实,大鼠外周血淋巴细胞离体照射诱导的γ-H2AX焦点形成数与大鼠整体照射的相吻合;而且γ射线照射诱导大鼠淋巴细胞γ-H2AX焦点数随照射剂量的增加而显著增加,与照射剂量之间呈线性关系,与文献报道的γ或X射线照射诱导人外周血淋巴细胞γ-H2AX焦点数与剂量之间呈线性关系相一致,其中有研究表明,在照射后2 h、0~1 Gy剂量范围内,其线性剂量-效应的斜率为4.05焦点/Gy[15],与本实验剂量-效应曲线斜率为6.81焦点/Gy的结果相接近,但存在一定的误差。分析其原因可能是γ-H2AX焦点计数标准不尽相同造成的,这种误差已被近年来多家实验室采用γ-H2AX焦点进行生物剂量估算比对所证实[15]。
[JP2] 综上所述,γ射线照射诱发大鼠和人外周血淋巴细胞γ-H2AX形成和消除动力学存在很好的一致性,辐射诱导pATM (S1981)或pDNA-PKcs(T2609)焦点与γ-H2AX焦点共定位比例相一致是大鼠和人淋巴细胞γ-H2AX焦点形成和消除动力学相同的重要原因;另外,大鼠淋巴细胞离体照射和大鼠整体照射诱导的γ-H2AX焦点形成数相一致,而且,γ-H2AX焦点形成数与照射剂量之间具有良好的线性关系。以上结果表明,大鼠为γ-H2AX作为辐射生物剂量计研究提供了切实可行的动物模型。
| [1] | Redon CE, Dickey JS, Bonner WM, et al. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin[J]. Adv Space Res, 2009, 43(8): 1171-1178. |
| [2] | Roch-Lefèvre S, Mandina T, Voisin P, et al. Quantification of γ-H2AX foci in human lymphocytes: a method for biological dosimetry after ionizing radiation exposure[J]. Radiat Res, 2010, 174(2): 185-194. |
| [3] | Rothkamm K, Horn S. γ-H2AX as protein biomarker for radiation exposure[J]. Ann Ist Super Sanita, 2009, 45(3): 265-271. |
| [4] | Goodarzi AA, Jeggo PA. Irradiation induced foci (IRIF) as a biomarker for radiosensitivity[J]. Mutat Res, 2012, 736(1-2): 39-47. |
| [5] | Rothkamm K, Lbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses[J]. Proc Natl Acad Sci USA, 2003, 100(9): 5057-5062. |
| [6] | Wang Z, Hu H, Hu M, et al. Ratio of γ-H2AX level in lymphocytes to that in granulocytes detected using flow cytometry as a potential biodosimeter for radiation exposure[J]. Radiat Environ Biophys, 2014, 53(2): 283-290. |
| [7] | Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation[J]. Nature, 2003, 421(6922): 499-506. |
| [8] | Davis AJ, Chen DJ. DNA double strand break repair via non- homologous end-joining[J]. Transl Cancer Res, 2013, 2(3): 130-143. |
| [9] | Beels L, Werbrouck J, Thierens H. Dose response and repair kinetics of γ-H2AX foci induced by in vitro irradiation of whole blood and T-lymphocytes with X- and γ-radiation[J]. Int J Radiat Biol, 2010, 86(9): 760-768. |
| [10] | Marková E, Torudd J, Belyaev I. Long time persistence of residual 53BP1/γ-H2AX foci in human lymphocytes in relationship to apoptosis, chromatin condensation and biological dosimetry[J]. Int J Radiat Biol, 2011, 87(7): 736-745. |
| [11] | Depuydt J, Baert A, Vandersickel V, et al. Relative biological effectiveness of mammography X-rays at the level of DNA and chromosomes in lymphocytes[J]. Int J Radiat Biol, 2013, 89(7): 532-538. |
| [12] | Vandersickel V, Beukes P, Van Bockstaele B, et al. Induction and disappearance of γ-H2AX foci and formation of micronuclei after exposure of human lymphocytes to 60Co γ-rays and p(66)+ Be(40) neutrons[J]. Int J Radiat Biol, 2014, 90(2): 149-158. |
| [13] | Redon CE, Nakamura AJ, Gouliaeva K, et al. The use of γ-H2AX as a biodosimeter for total-body radiation exposure in non-human primates[J]. PLoS One, 2010, 5(11): e15544. |
| [14] | Moroni M, Maeda D, Whitnall MH, et al. Evaluation of the γ-H2AX assay for radiation biodosimetry in a swine model[J]. Int J Mol Sci, 2013, 14(7): 14119-14135. |
| [15] | Rothkamm K, Horn S, Scherthan H, et al. Laboratory intercomparison on the γ-H2AX foci assay[J]. Radiat Res, 2013, 180(2): 149-155. |
| [16] | Burma S, Chen BP, Murphy M, et al. ATM phosphorylates histone H2AX in response to DNA double-strand breaks[J]. J Biol Chem, 2001, 276(45): 42462-42467. |
| [17] | Chen BP, Uematsu N, Kobayashi J, et al. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break[J]. J Biol Chem, 2007, 282(9): 6582-6587. |
| [18] | An J, Huang YC, Xu QZ, et al. DNA-PKcs plays a dominant role in the regulation of H2AX phosphorylation in response to DNA damage and cell cycle progression[J]. BMC Mol Biol, 2010, 11: 18. |
| [19] | Goodarzi AA, Noon AT, Deckbar D, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin[J]. Mol Cell, 2008, 31(2): 167-177. |
 2015, Vol. 35
2015, Vol. 35