Bone marrow-derived cells,which represent a heterogeneous cell population including haematopoietic stem cells (HSCs),mesenchymal stem cells (MSCs),bone marrow stromal cells (BMSCs),multipotent adult progenitor cells (MAPCs) and progenitor of endothelial cells (PECs),hold great promise in the cell therapy of many diseases and injuries[1, 2, 3]. Particularly,the bone marrow-derived cells play a very important role in the tissue repair and regeneration after radiation injury,but the underlying mechanism is still not fully clarified. The chimerism has been widely used to study the cell plasticity and differentiation capacity of bone marrow-derived cells. However,most studies on the chimerism were employed with lethal or sublethal dose radiation,which might lead to an increased death of animals and other complications[4]. In the present study,the engraftment of bone marrow-derived cells was investigated in syngeneic or allogeneic C57BL/6 mice receiving a lower dose of nonlethal radiation and the results showed that the bone marrow-derived cells were successfully grafted into various recipient tissues after irradiation with 2.5 Gy of whole body irradiation (WBI) in syngeneic mice,but not in allogeneic mice.
GFP+ transgenic mice were used as transplantation donors,purchased from the Model Animal Research Center of Nanjing University [strain code: C57BL/6-TgN(ActbEGFP)1Osb)][5]. Three-month-old wild-type C57BL/6 mice and BALB/c mice were used as recipients,which were obtained from Experimental Animals Center of Third Military Medical University. Animals were acclimated to standard laboratory conditions and kept in specific pathogen free (SPF) grade house. According to the animal care guidelines of the Third Military Medical University animal experimental operations were conduct.
2. Bone marrow preparationBone marrow was aseptically harvested from femurs and tibias of donor mice by flushing with DMEM/F12 medium combined with 10% fetal bovine serum (Hyclone,Beijing,China). Marrow samples were sufficiently pipetted,centrifuged at 1 000 revolutions per minute for 3 min. All the marrow cells were resuspended in DMEM/F12. For each recipient,a concentration about 1×107 viable nucleated cells per 300 μl volume was injected.
3. Irradiation and transplantationAll of 20 C57BL/6 mice and 20 BALB/c mice were used as transplantation recipients in the experiment. Those mice were housed in autoclaved cages and treated with antibiotics for 10 d before irradiation and 2 weeks after irradiation (with 280 mg erythromycin and 320 mg gentamicin sulfate per liter of deionized drinking water). Randomly selected 5 C57BL/6 mice were exposed to 2.5 Gy WBI from a 60Co source (Theratron-780 model,MDS Nordion,Ottawa,ON,Canada),while other 10 of the rest were divided equally into 6 and 8 Gy irradiation group. Within 3 h after transplantation,1×107 GFP bone marrow cells in a volume of 300 μl was transplanted via tail vein to the last 5 recipient mice as transplantation control group. BALB/c mice were treated the same as C57BL/6 group.
4. Tissue preparation and detection of donor's BMDCsFresh organic samples (bone marrow,lung,liver,spleen and small intestine) were fixed with 4% paraformaldehyde immediately,processed to paraffin wax blocks and cut into 4 μm sections. After dewaxing and rehydration,antigen retrieval was carried out at 96℃ for 10 min using sodium citrate solution (0.01 mol/L,pH 6.0). Activity of endogenous peroxidase was removed by incubation with 3% H2O2 for 15 min at room temperature,and nonspecific binding was blocked by 3% normal goat serum in incubator for 30 min at 37 ℃. Donor's BMDCs were identified by rabbit anti-GFP monoclonal antibody (1∶200,AG279,Beyotime,Hangzhou,China). Negative control was set with PBS. Horseradish peroxidase-(HRP-) conjugated goat anti-rabbit secondary antibody was used to bind the GFP antibody. Positive signals were shown by DAB response. Sections were counterstained with hematoxylin according to standard protocols after immunohistochemical (IHC) reaction[5].
All mice were irradiated by γ-ray irradiation as described from a 60Co source and were kept in SPF conditions. The bone marrow cells from GFP+ transgenic donor mice were infused within 3 h after the recipient mice were irradiated. The bone marrow,lung,liver,spleen and small intestine tissues were harvested at 120 d after transplantation. The preparation of tissue sections and IHC staining were performed as described previously[6]. Although GFP+ cells can be directly detected with a fluorescent microscope,the intensity of the signal was more evident by IHC staining. The results indicated that the GFP positive hematopoietic cells were successfully grafted in the bone marrow of syngeneic C57BL/6 recipient mice receiving a nonlethal 2.5 Gy whole body irradiation and in the allogeneic BALB/c recipient mice receiving 8 Gy (Figure 1). However,the GFP positive bone marrow-derived cells from C57BL/6 mice were not detected in the bone marrow of BALB/c recipient mice received 2.5 Gy irradiation.
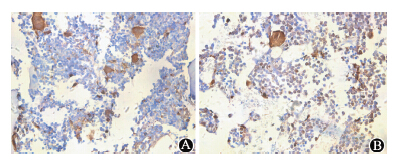 | Figure 1 Histological analysis of cell engraftment in the bone marrow ×400 A. Chimerism in medulla of C57BL/6 mice from 2.5 Gy radiation group at 120 d; B. Chimerism in medulla of BALB/c mice from 8 Gy radiation group at 120 d |
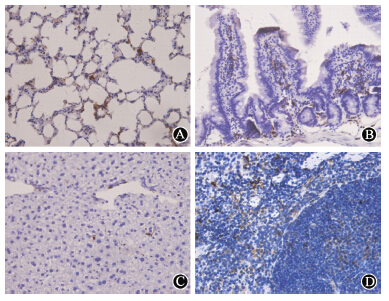 | Figure 2 Histological analysis of cell engraftment in different organs×400 at 120 d after donor cell transplantation,GFP+ bone marrow-derived cells were detected in (A) lung,(B) small intestine,(C) liver and (D) spleen |
At 120 d after transplantation,GFP+ cells were detected in the lung,small intestine,liver and spleen. The GFP+ cells accumulated in the lung interstitium,but not in the alveolar lumen. Most GFP+ cells migrated into the intestinal villi,and grafted in the lamina propria around small intestinal epithelial crypts,but no positive cells were detected in the epithelial lining. Compared to lethal dose irradiation,less positive cells differentiated into intestinal epithelial cells in the 2.5 Gy irradiation model. Many GFP+ cells were also detected in the interstitial tissue of liver and some cells were adjacent to the interlobular veins or liver sinusoids. However,rare GFP positive hepatic oval cells were incorporated into liver tissue. Additionally,a vast majority of GFP+ cells were detected in the spleen. Furthermore,the chimerism was also detected in different organs at various time-points (10,20,60,and 120 d) after transplantation (Figure 3). As an example,GFP+ bone marrow cells could be grafted into liver tissue at all-time points and the black arrows indicated significant GFP positive cells.
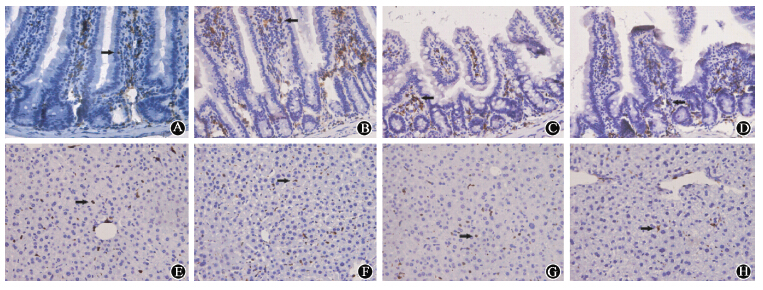 | Figure 3 Histological analysis of cell engraftment in the small intestine and liver at different time-points ×400 In 2.5 Gy irradiation model,GFP+ cells were incorporated into small intestinal and liver at 10 d (A,E),20 d (B,F),60 d (C,G),and 120 d (D,H) |
To further study the long-term engraftment of the allogeneic cells in recipient tissues,bone marrow cells from C57BL/6 GFP+ transgenic mice were also infused to irradiated BALB/c mice though tail vein. The results showed that the GFP+ cells were not detected in any tissue in mice received 2.5 or 6 Gy nonlethal irradiation,but the positive cells were detected in the lung,liver,intestinal and spleen in mice with 8 Gy lethal irradiation (Figure 4),suggested that the chimerism was dependent on both radiation dose and host genetic background at least.
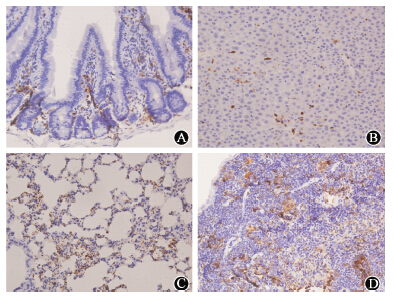 | Figure 4 Bone marrow-derived cells from GFP transgenic C57BL/6 mice were grafted in the small intestine(A),liver (B),lung (C),and spleen (D) in BALB/c mice receiving 8 Gy lethal irradiation |
Bone marrow-derived cells play very important roles in the repair and regeneration of damaged tissues and organs after radiation injury. It has been reported that irradiated mice with lethal dose can recover after transplantation of bone marrow cells from healthy mouse. Buckner et al[7] successfully performed the first clinical allogeneic bone marrow transplantation in a patient with leukemia. Because radiation could induce the damage of multiple tissues and organs,the mechanisms on the engraftment and therapeutic effects of the bone marrow-derived cells warrant further characterization. Chimera model provides an available tool for the mechanistic studies and helps to understand the plasticity and differentiation capability of transplanted cells.
Researchers used to establish chimera model by exposure of experimental animals to lethal or sublethal radiation[8, 9, 10, 11],but may lead to increased mortality,whole body damage and severe side-effects. Whether a lower radiation dose can successfully induced peripheral and organic chimerism is an interesting issue. In this study,we aimed to investigate the engraftment of bone marrow-derived cells in syngeneic BALB/c or allogeneic C57BL/6 mice received a lower dose of nonlethal radiation. The bone marrow-derived cells from GFP transgenic C57BL/6 mice were infused into syngeneic recipient mice received 2.5 Gy whole body irradiation and the transplanted cells were successfully grafted not only in haematopoietic tissues but also in multiple non-haemtopoietic tissues which was similar to the result with lethal and sublethal irradiation from others[9]. The GFP+ cells were detected in the host liver,lung,small intestine and spleen at even 120 d after transplantation,which indicated a successful long-term chimerism.
In the present study,we investigated the effects of radiation dosages and the genetic backgrounds of host mice on the engraftment of bone marrow-derived cells. The results showed a successful long-term engraftment of GFP+ bone marrow cells in syngeneic C57BL/6 mice,but not in the allogeneic BALB/c mice received 2.5 Gy irradiation. However,the successful chimerism was established in allogeneic BALB/c mice received 8 Gy lethal irradiation,which indicated that the radiation dose and host genetic background were important factors that affected the cell engraftment. Colvin et al[12] also studied the stem cell homing in syngeneic models and suggested that the chimerism appeared rapidly (<1 h) and was dependent on cellular adhesion and migration factors. Another important issue was the phenotype of the engrafted cells and their potential roles in tissue repair. It has been reported that bone marrow-derived cells can exhibit the properties of various cell types in the tissues of recipients through either cell fusion or transdifferentiation. This study also showed that bone marrow-derived cells could significantly grafted and repopulated the recipient's intestinal epithelium in 8 Gy irradiation model[6],while less cells were detected in the intestinal epithelium in 2.5 Gy irradiation model,suggested that radiation dose could modulate the fate of the engrafted cells. In summary,the establishment of syngeneic chimerism model of 2.5 Gy irradiation may provide a new approach to further study the roles and mechanisms of bone marrow-derived cells in response to radiation related injuries.
| [1] | Levine P, McDaniel K, Francis H, et al. Molecular mechanisms of stem cell therapy in alcoholic liver disease[J]. Dig Liver Dis, 2014, 46(5): 391-397. |
| [2] | King A, Balaji S, Keswani SG, et al. The role of stem cells in wound angiogenesis[J]. Adv Wound Care (New Rochelle), 2014, 3(10): 614-625. |
| [3] | Qu B, Xin GR, Zhao LX, et al. Testing stem cell therapy in a rat model of inflammatory bowel disease: role of bone marrow stem cells and stem cell factor in mucosal regeneration[J]. PLoS One, 2014, 9(10): e107891. |
| [4] | Okabe M, Ikawa M, Kominami K, et al. 'Green mice' as a source of ubiquitous green cells[J]. FEBS Lett, 1997, 407(3): 313-319. |
| [5] | Liu D, Wang F, Zou Z, et al. Bone marrow derivation of interstitial cells of cajal in small intestine following intestinal injury[J]. J Biomed Biotechnol, 2010, 2010(2010): 164986. |
| [6] | Liu D, Wang F, Zou Z, et al. Long-term repopulation effects of donor BMDCs on intestinal epithelium[J]. Dig Dis Sci, 2010, 55(8): 2182-2193. |
| [7] | Buckner CD, Epstein RB, Rudolph RH, et al. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. 1970[J]. J Hematother Stem Cell Res, 2001, 10(2): 201-208. |
| [8] | Singh V, Jaini R, Torricelli AA, et al. A method to generate enhanced GFP+ chimeric mice to study the role of bone marrow-derived cells in the eye[J]. Exp Eye Res, 2013, 116: 366-370. |
| [9] | Filip S, Mokrý J, Vávrová J, et al. The peripheral chimerism of bone marrow-derived stem cells after transplantation: regeneration of gastrointestinal tissues in lethally irradiated mice[J]. J Cell Mol Med, 2014, 18(5): 832-843. |
| [10] | Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts[J]. Nature, 1984, 307(5947): 168-170. |
| [11] | Lubin I, Faktorowich Y, Lapidot T, et al. Engraftment and development of human T and B cells in mice after bone marrow transplantation[J]. Science, 1991, 252(5004): 427-431. |
| [12] | Colvin GA, Lambert JF, Dooner MS, et al. Murine allogeneic in vivo stem cell homing[J]. J Cell Physiol, 2007, 211(2): 386-391. |
 2015, Vol. 35
2015, Vol. 35


