2. Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, Washington DC, USA;
3. The Feinstein Institute for Medical Research, Manhasset, NY, USA
High mobility group box 1(HMGB1),belonging to the high-mobility group (HMG) protein family,is a cytokine-like 215 amino acid nuclear protein,which is an important mediator of the body's inflammatory,ischemia and injury[1, 2]. It is known that HMGB1 is structured into three domains,two basic HMG boxes (HMG domains A and B) and a highly acidic C-terminal domain,which confer an overall dipolar appearance to this protein. Each of the HMG boxes is formed by two short and one long α-helix that upon folding produce an L- or V-shaped three-dimensional domain structure. The concave surface of the L- or V-shaped HMG box domain contacts the DNA in the minor groove in two slightly different ways introducing important modifications in the structure of DNA,in particular a strong bend[1, 2]. Presumably,these features are of relevance for the biological functions in which HMGB1 has been involved (DNA repair,recombination,replication,and transcription)[1, 2]. HMGB1 can interact through its HMG box domains with a broad range of proteins ranging from nuclear cell proteins to viral proteins,including the recombination activation gene protein (RAG1),several transcription factor proteins including the tumor suppressor p53,the octamer transcription factors Oct1,Oct2,Oct4 and Oct6,some homeotic (Hox) proteins,the steroid receptors,the general initiation factor human TATA-binding protein (hTBP),and the viral replication proteins Rep78 and Rep68[1, 2, 3, 4, 5, 6]. HMG box A is important for binding to hTBP and p53,whereas the binding to Oct factors,Hox factors,and hormone receptors can take place through boxes A or B[3, 4]. HMGB1 is a critical target for cancer therapy[7].
Exposure of cancer cells to ionizing radiation resulted in the arrest of cell cycle progression,down- and up-expression of the transcription of specific genes,modification of nucleosomal structure,and activation of the DNA repair machinery. An important role was found for histone acetylation and deacetylation in the modification of chromatin structure and in monitoring chromosomal integrity[8]. A number of proteins that participate in regulation of the cell cycle and transcription of cancer cells are associated with histone acetylation or deacetylation activities[9, 10],including p300/CBP-associated factor(pCAF),breast cancer susceptibility gene BRCA1/2,ataxia telangiectasia mutated(ATM),and ATM-like proteins. The product of the retinoblastoma gene (Rb) represses transcription of the E2F gene by recruiting the mammalian deacetylase proteins histone deacetylase1(HDAC1) and histone deacetylase2(HDAC2),to which it binds through an LXCXE motif in its pocket domain[11]. An LXCXE motif in the HDAC1 protein was required for binding of HDAC1 to ATM[12].
Previous studies suggested that enforced expression of HMGB1 increased radiosensitivity of breast cancer cells,in which an interaction was not required[13]. Similar findings were obtained for other members of HMGB family,HMGB2,3 and 4[14]. These results indicate an unknown mechanisms contributing to effects of HMGB1-mediatied radiosensitivity. In this study,the results demonstrated that HMGB1 interacted with HDAC1 both in vitro and in vivo and that the increased extent of the HMGB1-HDAC1 association was found in the cells exposed to ionizing radiation. HDAC1 is critical for HMGB1 radiosensitization,as evidenced by an application of a mutant LXCXE motif and a specific HMGB1 inhibitor.
Human breast cancer lines MDA-MB-231 and MDA-MB-468 were maintained in DMEM supplemented with 5% fetal calf serum,100 U/ml penicillin and 100 μg/ml streptomycin at 37℃ in a 5% carbon dioxide atmosphere. Exponentially growing cells were exposed to γ-ray-irradiation (J.L. Shepherd Mark I Radiator) with a 137Cs source emitting at a fixed dose rate of 2.5 Gy/min. The trichostatin A (TSA) was purchased from BioVision,Inc.,USA.
2. Methyl thiazolyl tetrazolium (MTT) survival assayMTT survival assay was performed as described previously[12, 13]. MTT assay is a commonly used method in evaluation of cell survival,based on the ability of viable cells to convert MTT,a soluble tetrazolium salt [3-(4,5-dimethylthuazole-2-yl)-2,5 diphenyl tetrazolium bromide],into an insoluble formazan precipitate,which is quantitated by sepectrophotometry following solubilization in dimethyl sulfoxide (DMSO). Briefly,cells (5×105 cells/ml) were plated in 60 mm Patri dishes with 2 ml of medium,allowed to attach for 30 h and irradiated. Untreated and treated cells were incubated with MTT (0.5 mg/ml) for 4 h. Then the cells were then solubilized in DMSO and transferred into each well in 96-well Patri dishes (10 wells for each treatment). Absorbance readings were taken using a Dynatech 96-well spectrophotometer. The amount of MTT dye reduction was calculated based on the difference between absorbance at 570 and 630 nm. Cell viability in treated cells was expressed as the amount of dye reduction relative to that of untreated control cells. Four sets of independent experiments were performed.
3. Clonogenic assayThe cells were trypsinized immediately after irradiation and counted. Known numbers were sub-cultured in 100 mm culture dishes in 2 sets of triplicates at each dose of irradiation. Sufficient numbers were seeded to ensure that about 50-100 macroscopic colonies would appear in each plate of un-irradiated and un-transfected control cells at the end of 21 d. Colonies were then fixed,stained and counted. Surviving fractions were normalized by the plating efficiency of un-irradiated controls (50% for MDA-MB-231 and MDA-MB-468).
4. HMGB 1 expression vectorThe wild-type HMGB1 expression plasmid (wtHMGB1) was created by cloning the full-length HMGB1 cDNA into a mammalian expression vector pCMV-Tag2B (Invitrogen,USA). LXCXE-defective HMGB1 (HMGB1-RXRXH),in which the LXCXE changed into the RXRXH expression vectors was created by modification of the wtHMGB1 cDNA in the pCMVTag2B vector by a MORPH site-directed plasmid DNA mutagenesis kit (Stratagene,USA).
5. Transient transfection assayExponentially growing cells in 100 mm Petri tissue culture dishes at about 70% confluence were incubated overnight with 5 μg plasmid DNA,using Lipofectamine 2000 (Invitrogen,USA),according to the manufacturer's instructions. The transfected cells were incubated in medium containing G418 (0.5 mg/ml),the G418-resistant colonies were observed approximately 3 weeks after transfection. Pooled G418-resistant colonies were used for subsequent experiments.
6. Glutathione S-transferase (GST)-HMGB1 constructsAn expression vector encoding a GST fusion protein containing HGMB1 (GST-HMGB1) was generated by inserting the corresponding polymerase chain reaction-generated full-length of human HMGB1 cDNA into pGEX-4T-1(Kodak,Japan). A cDNA encoding a mutant fusion protein (GST-HMGB1/C106F) in which Cys 106 of the LXCXE motif of HMGB1 was replaced by phenylalanine was generated from GST-HMGB1 cDNA with the use of a QuickChange site-directed mutagenesis kit (Stratagene,USA). All fusion proteins were produced in and purified from Escherichia coli which were described in the previous studies[12, 13].
7. GST pull-down assayThe 1.4-kb full-length human HDAC1 cDNA incorporated into the pcDNA3 vector (Invitrogen,USA) was subjected to in vitro transcription and translation in the presence of 35S-methionine with a TNT T7-coupled transcription and translation kit (Promega,USA). Beads coated with GST fusion proteins (10 μg) were incubated for 4 h at 4℃ with in vitro translated 35S-labeled HDAC1 (10 μg) or nuclear extracts (750 μg of protein) in a final volume of 400 μl containing TNN buffer (40 mmol/L Tris-HCl (pH 8.0),120 mmol/L NaCl,0.5% (V/V) Nonidet P-40,and protease inhibitors). After extensive washing of the beads,bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDS-PAGE) and either autoradiography,as described in the previous studies[12, 13].
8. Immunoprecipitation and immunoblot analysisNuclear extracts were prepared as described[12, 13],and the concentration of protein was determined with the Bradford reagent (Bio-Rad). The extracts (1 mg of protein) were subjected to immunoprecipitation for 6 h at 4℃ with an anti-HDAC1 antibody to a final volume of 20 μl of TNN buffer. After the addition of protein A/G-agarose (Santa Cruz Biotechnology,USA),the reaction mixtures were incubated for an additional 2 h. The immunoprecipitates were washed extensively and subjected to SDS-PAGE,and the separated proteins were then transferred to a nitrocellulose membrane and subjected to Western blot analysis as described[12, 13]. Preliminary antibodies for Western blot were a mouse monoclonal anti-HADC1 antibody (Millipore,USA) and a mouse monoclonal anti-HMGB1 antibody (R&D Systems,USA).
9. Histone deacetylase activity assayHistone deacetylase activity assay was completed by a histone deacetylase assay kit (Millipore,USA) according to the manufacturer's protocol. All samples were assayed in triplicate.
10. Statistical analysisThe results were presented in the form of x±s. The statistical analysis was performed by SPSS 13.0. The difference between groups was processed by t-test. P<0.05 is considered significantly different.
Previous studies showed that enhanced expression of HMGB1 increased sensitivity of breast cancer cells to ionizing radiation[12, 13] and chemotherapeutic agents[14, 15, 16]. The impact of HMGB1 was also examined on susceptibility of human breast cancer MDA-MB-231 and MDA-MB-468 cells to ionizing radiation. Human breast cancer cells were transiently transfected with a pCMV-Tag2B expression vector carried with a full-length of HMGB1 cDNA (pCMV-Tag2B/wtHMGB1) or an 'empty' pCMV-Tag2B (as the control). The stable transfactents were then subjected to MTT assay 24 h or colony formation assay following irradiation with γ-rays. The HMGB1 protein level of transfectants was monitored by Western blot assay (Figure 1),showing that the expression of HMGB1 protein was highly detected in pCMV-Tag2B/wtHMGB1 transfected cells compared with the control pCMV-Tag2B transfected cells. With increased expression of HMGB1,a higher radiosensitivity was observed in MDA-MB-231 transfectants with wtHMGB1 (ID50=1.8 Gy) than that in MDA-MB-231 transfectants with pCMV-Tag2B (ID50=3.5 Gy) (t=2.21,P<0.05),as shown with colony formation assays in Figure 1. Similar results were also obtained with MDA-MB-468 cells,i.e.,ID50=2.1 Gy (wtHMGB1 transfectants) vs. ID50=4.0 Gy (pCMV-Tag2B transfectants) (t=2.19,P<0.05). The data with MTT cell viability assay were consistent with these results observed by colony formation assay. These results were consistent with the previous studies in other breast cancer cell lines[12, 13],indicating that HMGB1 was indeed able to modulate radiosensitivity of breast cancer cells by increasing cellular susceptibility of breast cancer to ionizing radiation.
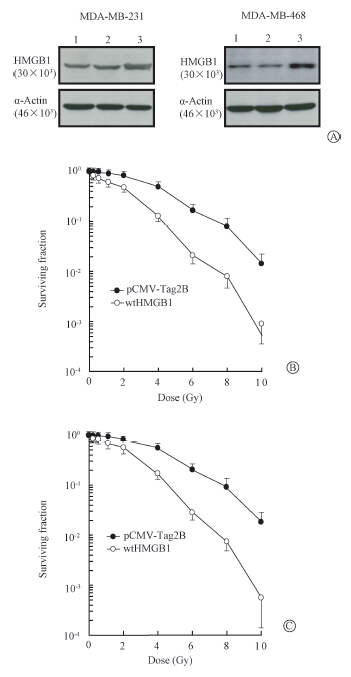 | Note:1.Control; 2.pCV-Tag2B; 3. wtHMGB1Figure 1 HMGB1 radiosensitization,proliferating cultures of transfects were harvested for the expression of HMGB1 protein by Western blot assay (A) or exposed to indicated doses of γ-rays and harvested for clonogenic assays in MDA-MB-231(B) and MDA-MB-468(C) transfectants |
HDAC1 plays an important role in cell response to DNA damages caused by ionizing radiation[17, 18, 19]. Inhibition of HDAC1 activity by specific HDAC inhibitors increases radiosensitivity of cancer cells,including breast cancer[20]. Several proteins,such as ATM,associate with HDAC1 through an LXCXE binding motif and mediates HDAC1 activities[21]. Sequence analysis has revealed an LXCXE motif (amino acids 104-108) presents at the center region of HMGB1 protein[12, 13]. To investigate the possible role of HDAC1 in HMGB1 radiosensitization,protein-protein binding assays was first performed in vitro by GST capture assays. In vitro translated 35S-labeled HDAC1 was incubated with beads coated with a bacterially produced GST fusion protein containing a full-length (amino acids 1-215) of HMGB1 (GST-wtHMGB1). Beads coated with the 'empty' GST fusion proteins were used as negative controls. The 35S-labeled HDAC1 bound to the GST-HMGB1 protein but not to the 'empty' GST fusion protein. The extent of GST-HMGB1/C106F interaction with HDAC1 was dramatically reduced to be almost undetectable relative to that observed with the GST-wtHMGB1. Furthermore,HMGB1 with the LXCXE mutant lost wtHMGB1 capability to cause a significant increase of radiosensitivity (Figure 2). Therefore,these data indicate that the LXCXE motif was essential in the HMGB1 binding to HDAC1 and required for HMGB1 radiosensitization.
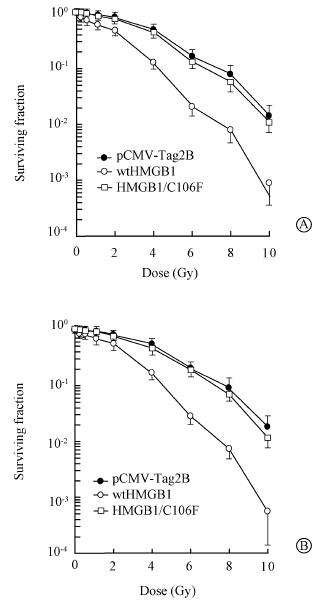 | Figure 2 Cell survival after exposure of radiation at inducated doses by clonogenic formation assay in MDA-MB-231(A) and MDA-MB-468(B) transfectants |
It was also determined whether the DNA damages induced by ionizing radiation would affect the interaction of HMGB1 with HDAC1. The human breast cancer MDA-MB-468 cells were exposed to γ-ray irradiation with a single dose of 5 Gy and then subjected to immunoprecipitation-Western blot at various intervals following irradiation. As shown in Figure 3,the HMGB1 protein was indeed detected in the immunoprecipitates prepared from the nuclear extracts with antibodies to HDAC1 under the condition of un-irradiation. MDA-MB-231 cells were exposed to ionizing radiation (20 Gy),and at the times indicated below,nuclear extracts were prepared and incubated with beads coated with GST-HMGB1. Proteins that bound to the beads were subjected to SDS-PAGE on a 7% gel and Western blot assay with antibodies to HDAC1. A portion of the nuclear extract from un-irradiated cells corresponding to 10% of the input to the binding reaction mixture was also directly subjected to immunoblot analysis.The increased amount of HMGB1 present in the immunoprecipitates was observed post-irradiation. In contrast,the HMGB1 protein was not detectable in the immunoprecipitates with a mouse IgG antibody (as the control). These results further demonstrated that HMGB1 directly binds to HDAC1 in vivo and such association was increased by exposure to irradiation.
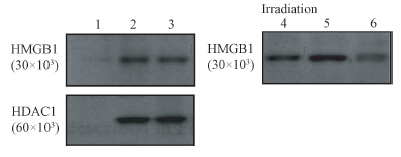 | Note:1.Normal IgG; 2.MDA-MD-231; 3.MDA-MB-468;4.10% input; 5.+; 6.-Figure 3 Endogenous association of HMGB1 and HDAC1 evidenced by immunoprecipitation-Western blot assay. The association was increased in the presence of irradiation. A mouse IgG immunoprecipitation from MDA-MB-231 cells was used as negative control |
To further investigate the effect of HDAC1 on HMGB1 radiosensitization,a specific HDAC1 inhibitor trichostatin A (TSA) was employed. Consistent with the data shown in Figure 1,enhanced expression of HMGB1 by transient transfection resulted in an increased radiosensitivity in both of MDA-MB-231 and MDA-MB-468 cell lines (Figure 4). The HMGB1 radiosensitization in these two cell lines was highly elevated in the presence of trichostatin A,although trichostatin A at this dose did not cause any effect on the cellular susceptibility to ionizing radiation. Similar data were observed in other cell lines.
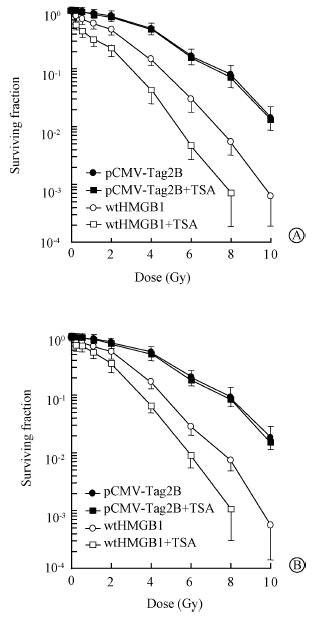 | Figure 4 A critical role of HDAC1 for HMGB1 radiosensitization. Proliferating cultures of MDA-MB-231(A) and MDA-MB-468(B) transfects were exposed to indicated doses of γ-rays,incubated in the medium containing trichostatin A harvested for colony formation assays |
To determine whether the HDAC1 associated with HMGB1 exhibits histone deacetylase activity,we incubated nuclear extracts of MDA-MB-231 cells with beads coated with GST-HMGB1 and then assayed the bead-associated proteins for deacetylase activity. Such beads showed a high level of deacetylase activity,which was inhibited by treatment with TSA. Beads coated with GST,GST-p300,or GST-HMGB1/C106F retained much less histone deacetylase activity after incubation with MDA-MB-231 nuclear extracts than GST-HMGB1-coated beads. Furthermore,the amount of HMGB1-associated histone deacetylase activity was increased after exposure of MDA-MB-231 cells to ionizing radiation,as revealed by GST-HMGB1 precipitation and immunoprecipitation assays (Figure 5); immunoprecipitates prepared with an irrelevant antibody did not exhibit histone deacetylase activity.
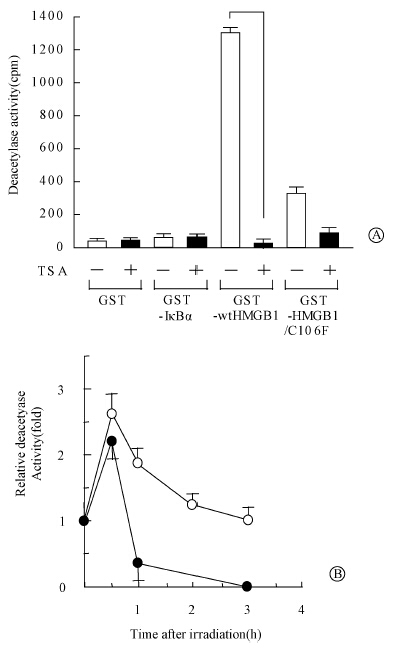 | Figure 5 Association of HMGB1 with histone deacetylase activity A. beads coated with the indicated GST fusion proteins were incubated with nuclear extracts of MDA-MB-231 cell; B. nuclear extracts prepared from MDA-MB-231 cells at the indicated times after irradiation were either subjected to immunoprecipitation with antibodies to HMGB1 or incubated with beads coated with GST-HMGB1 |
The present studies indicate that HMGB1 increases sensitivity of breast cancer cell lines MDA-MB-231 and MDA-MB-468 to ionizing radiation,which is consistent with our previous studies in other breast cancer cell lines,MCF-7,T47D and BT-549[12, 13]. These findings indicate that HMGB1 is an important mediator of radiosensitivity in breast cancer cells. No significant roles of Rb and p53 are suggested in the HMGB1 radiosensitization,since the radiosensitization is observed in a wide number of breast cancer cell lines with different Rb and p53 status. Moreover,HMGB1 directly binds to HDAC1,the association requires the LXCXE motif of HMGB1 and is increased by irradiation. The HDAC1 promoter activity is significantly repressed by wtHMGB1,while not by HMGB1 with mutation of LXCXE motif. In addition,the HMGB1 radiosensitization is lost or significantly decreased by the mutant LXCXE motif of HMGB1 and in the presence of HDAC1 inhibitor. These results indicate that HDAC1 may play an important role in HMGB1 mediated radiosensitivity in breast cancer cells,no matter the effect of other HDAC1 binding proteins,such as ATM,Rb on HMGB1-HDAC1 interaction needs to be further studied. This research with breast cancer cells indicate that mutations in the HMGB1 gene affect the interaction between HMGB1 and HDAC1 and may thereby prevent the increase in histone deactylase activity after irradiation. This observation is consistent with previous studies showing that HMGB1 is associated with chromatin and that decondensation of chromatin increases the radiosensitivity of DNA with respect to formation of double-strand breaks[22]. Therefore,it is also possible that breast cancer cells show an increased susceptibility to radiation-induced DNA damage because of the HMGB1 binding to HDAC1 as a key regulator of histone deactylase activity.
| [1] | Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis[J]. Expert Opin Ther Targets, 2014, 18(3): 257-268. |
| [2] | Zhu S, Li W, Ward MF, et al. High mobility group box 1 protein as a potential drug target for infection- and injury-elicited inflammation[J]. Inflamm Allergy Drug Targets, 2010, 9(1): 60-72. |
| [3] | Dintilhac A, Bernués J. HMGB1 interacts with many apparently unrelated proteins by recognizing short amino acid sequences[J]. J Biol Chem, 2002, 277(9): 7021-7028. |
| [4] | Imamura T, Izumi H, Nagatani G, et al. Interaction with p53 enhances binding of cisplatin-modified DNA by high mobility group 1 protein[J]. J Biol Chem, 2001, 276(10): 7534-7540. |
| [5] | Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling[J]. Mol Med, 2008, 14(7-8): 476-484. |
| [6] | Sims GP, Rowe DC, Rietdijk ST, et al. HMGB1 and RAGE in inflammation and cancer[J]. Annu Rev Immunol, 2010, 28: 367-388. |
| [7] | Lotze MT, DeMarco RA. Dealing with death: HMGB1 as a novel target for cancer therapy[J]. Curr Opin Investig Drugs, 2003, 4(12): 1405-1409. |
| [8] | Parbin S, Kar S, Shilpi A, et al. Histone deacetylases: a saga of perturbed acetylation homeostasis in cancer[J]. J Histochem Cytochem, 2014, 62(1): 11-33. |
| [9] | Ho AS, Turcan S, Chan TA. Epigenetic therapy: use of agents targeting deacetylation and methylation in cancer management[J]. Onco Targets Ther, 2013, 6: 223-232. |
| [10] | Barneda-Zahonero B, Parra M. Histone deacetylases and cancer[J]. Mol Oncol, 2012, 6(6): 579-589. |
| [11] | Brehm A, Miska EA, McCance DJ, et al. Retinoblastoma protein recruits histone deacetylase to repress transcription[J]. Nature, 1998, 391(6667): 597-601. |
| [12] | Jiao Y, Wang HC, Fan SJ. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer[J]. Acta Pharmacol Sin, 2007, 28(12): 1957-1967. |
| [13] | Wang LL, Meng QH, Jiao Y, et al. High-mobility group boxes mediate cell proliferation and radiosensitivity via retinoblastoma-interaction-dependent and -independent mechanisms[J]. Cancer Biother Radiopharm, 2012, 27(5): 329-335. |
| [14] | Pan B, Chen D, Huang J, et al. HMGB1-mediated autophagy promotes docetaxel resistance in human lung adenocarcinoma[J]. Mol Cancer, 2014, 13:165. |
| [15] | Liu L, Yang M, Kang R, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells[J]. Leukemia, 2011, 25(1): 23-31. |
| [16] | Kawahara N, Tanaka T, Yokomizo A, et al. Enhanced coexpression of thioredoxin and high mobility group protein 1 genes in human hepatocellular carcinoma and the possible association with decreased sensitivity to cisplatin[J]. Cancer Res, 1996, 56(23): 5330-5333. |
| [17] | Nagaki S, Yamamoto M, Yumoto Y, et al. Non-histone chromosomal proteins HMG1 and 2 enhance ligation reaction of DNA double-strand breaks[J]. Biochem Biophys Res Commun, 1998, 246: 137-141. |
| [18] | Ugrinova I, Zlateva S, Pashev IG, et al. Native HMGB1 protein inhibits repair of cisplatin-damaged nucleosomes in vitro[J]. Int J Biochem Cell Biol, 2009, 41(7): 1556-1562. |
| [19] | Tang D, Loze MT, Zeh HJ, et al. The redox protein HMGB1 regulates cell death and survival in cancer treatment[J]. Autophagy, 2010, 6(8): 1181-1183. |
| [20] | Zhang B, Wang Y, Pang X. Enhanced radiosensitivity of EC109 cells by inhibition of HDAC1 expression[J]. Med Oncol, 2012, 29(1): 340-348. |
| [21] | Kim GD, Choi YH, Dimtchev A, et al. Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase[J]. J Biol Chem, 1999, 274(44): 31127-31130. |
| [22] | Anuranjani, Bala M. Concerted action of Nrf2-ARE pathway, MRN complex, HMGB1 and inflammatory cytokines-implication in modification of radiation damage[J]. Redox Biol, 2014, 2: 832-846. |
 2015, Vol. 35
2015, Vol. 35


